
Ķعż³Įµķ£Ńõ»Æ·Ø“¦Ąķŗ¬øõ·ĻĖ®£¬¼õÉŁ·ĻŅŗÅŷŶŌ»·¾³µÄĪŪČ¾£¬Ķ¬Ź±»ŲŹÕK2Cr2O7”£ŹµŃéŹŅ¶Ōŗ¬øõ·ĻŅŗ(ŗ¬ÓŠCr3£«”¢Fe3£«”¢K£«”¢SO ”¢NO
”¢NO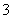 ŗĶÉŁĮæCr2O
ŗĶÉŁĮæCr2O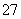 )»ŲŹÕÓėŌŁĄūÓĆ¹¤ŅÕČēĻĀ£ŗ
)»ŲŹÕÓėŌŁĄūÓĆ¹¤ŅÕČēĻĀ£ŗ

ŅŃÖŖ£ŗ¢ŁCr(OH)3£«OH£===CrO £«2H2O£»
£«2H2O£»
¢Ś2CrO £«3H2O2£«2OH£===2CrO
£«3H2O2£«2OH£===2CrO £«4H2O£»
£«4H2O£»
¢ŪH2O2ŌŚĖįŠŌĢõ¼žĻĀ¾ßÓŠ»¹ŌŠŌ£¬Äܽ«£«6¼ŪCr»¹ŌĪŖ£«3¼ŪCr”£
(1)ŹµŃéÖŠĖłÓĆKOHÅضČĪŖ6 mol·L£1£¬ĻÖÓĆKOH¹ĢĢåÅäÖĘ250 mL 6 mol·L£1µÄKOHČÜŅŗ£¬³żÉÕ±”¢²£Į§°ōĶā£¬»¹±ŲŠčÓƵ½µÄ²£Į§ŅĒĘ÷ÓŠ________”£
(2)ĀĖŅŗ¢ńĖį»ÆĒ°£¬½ųŠŠ¼ÓČȵÄÄæµÄŹĒ________”£±łŌ””¢¹żĀĖŗó£¬Ó¦ÓĆÉŁĮæĄäĖ®Ļ“µÓK2Cr2O7£¬ĘäÄæµÄŹĒ________________________”£
(3)ĻĀ±ķŹĒĻą¹ŲĪļÖŹµÄČܽā¶ČŹż¾Ż£ŗ
| ĪļÖŹ | 0 ”ę | 20 ”ę | 40 ”ę | 60 ”ę | 80 ”ę | 100 ”ę |
| KCl | 28.0 | 34.2 | 40.1 | 45.8 | 51.3 | 56.3 |
| K2SO4 | 7.4 | 11.1 | 14.8 | 18.2 | 21.4 | 24.1 |
| K2Cr2O7 | 4.7 | 12.3 | 26.3 | 45.6 | 73.0 | 102.0 |
| KNO3 | 13.9 | 31.6 | 61.3 | 106 | 167 | 246.0 |
øł¾ŻČܽā¶ČŹż¾Ż£¬²Ł×÷¢ń¾ßĢå²Ł×÷²½ÖčĪŖ¢Ł__________________”¢¢Ś__________”£
(4)³ĘČ”²śĘ·ÖŲøõĖį¼ŲŹŌŃł2.000 gÅä³É250 mLČÜŅŗ£¬Č”³ö25.00 mLӌ׶ŠĪĘæÖŠ£¬¼ÓČė10 mL 2 mol·L£1 H2SO4ŗĶ×ćĮæµā»ÆÄĘ(øõµÄ»¹Ō²śĪļĪŖCr3£«)£¬·ÅÓŚ°µ“¦5 min£¬Č»ŗó¼ÓČė100 mLĖ®£¬¼ÓČė3 mLµķ·ŪÖøŹ¾¼Į£¬ÓĆ0.120 0 mol·L£1 Na2S2O3±ź×¼ČÜŅŗµĪ¶Ø(I2£«2S2O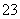 ===2I££«S4O
===2I££«S4O )”£
)ӣ
¢ŁČōŹµŃéÖŠ¹²ÓĆČ„Na2S2O3±ź×¼ČÜŅŗ30.00 mL£¬ĖłµĆ²śĘ·ÖŠÖŲøõĖį¼ŲµÄ“æ¶ČĪŖ________(ÉčÕūøö¹ż³ĢÖŠĘäĖūŌÓÖŹ²»²ĪÓė·“Ó¦)”£
¢ŚČōµĪ¶Ø¹ÜŌŚŹ¹ÓĆĒ°Ī“ÓĆNa2S2O3±ź×¼ČÜŅŗČóĻ“£¬²āµĆµÄÖŲøõĖį¼ŲµÄ“æ¶Č½«________(Ģī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°²»±ä”±)”£
“š°ø””(1)250 mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü
(2)³żČ„H2O2””³żČ„¾§Ģå±ķĆę²ŠĮōµÄŌÓÖŹ£¬¼õŠ”K2Cr2O7µÄĖšŗÄ
(3)¢ŁÕō·¢ÅØĖõ””¢Ś³ĆČČ¹żĀĖ
(4)¢Ł88.2%””¢ŚĘ«øß
½āĪö””(1)ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗ£¬ŠčŅŖÓƵ½ČŻĮæĘ棬ŌŚ¶ØČŻŹ±ŅŖÓƵ½½ŗĶ·µĪ¹Ü”£(2)ŌŚ¼ÓČČŹ±£¬H2O2»į·¢Éś·Ö½ā·“Ó¦”£ÓƱłĖ®Ļ“¾§ĢåŹĒĪŖĮĖ½µµĶ¾§ĢåµÄČܽā¶Č£¬Ōö¼Ó²śĀŹ”£(3)“ÓČܽā¶Č±ķÖŠŹż¾ŻÖŖ£¬K2SO4Čܽā¶Č±ČK2Cr2O7”¢KNO3Š”£¬²ÉÓĆÕō·¢ÅØĖõ£¬½«K2SO4Īö³ö¶ų³żČ„£¬³ĆČČ¹żĀĖµĆŗ¬ÓŠ“óĮæK2Cr2O7ŗĶÉŁĮæµÄKNO3µÄĀĖŅŗ£¬ŌŁ²ÉÓƱłĖ®½µĪĀ½į¾§£¬æÉĪö³öK2Cr2O7¾§Ģ唣
(4)¢ŁÕŅ³öµĪ¶ØµÄ¹ŲĻµ£ŗK2Cr2O7”«3I2”«6S2O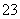 £¬m(K2Cr2O7)£½0.120 0 mol·L£1”Į30”Į10£3 L”Į294 g·mol£1”Į
£¬m(K2Cr2O7)£½0.120 0 mol·L£1”Į30”Į10£3 L”Į294 g·mol£1”Į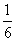 £½0.176 4 g£¬ÖŲøõĖį¼ŲµÄ“æ¶ČĪŖ(0.176 4”Į250/25)/2”Į100%£½88.2%”£¢ŚČōĪ“ÓĆNa2S2O3ČóĻ“µĪ¶Ø¹Ü£¬ŌņNa2S2O3µÄÅØ¶Č±äŠ”£¬ĻūŗĵÄĢå»ż±ä¶ą£¬Ōņ¼ĘĖć³öµÄI2ŅŌ¼°K2Cr2O7µÄĮæ½«Ę«“ó”£
£½0.176 4 g£¬ÖŲøõĖį¼ŲµÄ“æ¶ČĪŖ(0.176 4”Į250/25)/2”Į100%£½88.2%”£¢ŚČōĪ“ÓĆNa2S2O3ČóĻ“µĪ¶Ø¹Ü£¬ŌņNa2S2O3µÄÅØ¶Č±äŠ”£¬ĻūŗĵÄĢå»ż±ä¶ą£¬Ōņ¼ĘĖć³öµÄI2ŅŌ¼°K2Cr2O7µÄĮæ½«Ę«“ó”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijŠ”×éĶ¬Ń§ĪŖĢ½¾æH2O2”¢H2SO3”¢Br2µÄŃõ»ÆŠŌĒæČõ£¬Éč¼ĘČēĻĀŹµŃé(¼Š³ÖŅĒĘ÷ŅŃĀŌČ„£¬×°ÖƵÄĘųĆÜŠŌŅŃ¼ģŃé)”£
ŹµŃé¼ĒĀ¼ČēĻĀ£ŗ
| ŹµŃé²Ł×÷ | ŹµŃéĻÖĻó | |
| ¢ń | “ņæŖ»īČūa£¬µĪ¼ÓĀČĖ®£¬ ¹Ų±Õ»īČūa | AÖŠČÜŅŗ±äĪŖŗģ×ŲÉ« |
| ¢ņ | “µČėČČæÕĘų | AÖŠŗģ×ŲÉ«Ć÷ĻŌ±äĒ³£»BÖŠÓŠĘųÅŻ£¬²śÉś“óĮæ°×É«³Įµķ£¬»ģŗĻŅŗŃÕÉ«ĪŽĆ÷ĻŌ±ä»Æ |
| ¢ó | Ķ£Ö¹“µČėæÕĘų£¬“ņæŖ»īČūb£¬ÖšµĪ¼ÓČėH2O2ČÜŅŗ | æŖŹ¼Ź±ŃÕÉ«ĪŽĆ÷ĻŌ±ä»Æ£»¼ĢŠųµĪ¼ÓH2O2ČÜŅŗ£¬Ņ»¶ĪŹ±¼äŗ󣬻ģŗĻŅŗÖš½„±ä³Éŗģ×ŲÉ« |
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)AÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ________________________________________________________________________”£
(2)ŹµŃé²Ł×÷¢ņ“µČėČČæÕĘųµÄÄæµÄŹĒ________________________________________________________________________”£
(3)×°ÖĆCµÄ×÷ÓĆŹĒ__________£¬CÖŠŹ¢·ÅµÄŅ©Ę·ŹĒ______________________”£
(4)ŹµŃé²Ł×÷¢ó£¬»ģŗĻŅŗÖš½„±ä³Éŗģ×ŲÉ«£¬Ęä¶ŌÓ¦µÄĄė×Ó·½³ĢŹ½ŹĒ________________________________________________________________________”£
(5)ÓÉÉĻŹöŹµŃéµĆ³öµÄ½įĀŪŹĒ________________________________________________________________________”£
(6)ŹµŃé·“Ė¼£ŗ
¢ŁÓŠĶ¬Ń§ČĻĪŖŹµŃé²Ł×÷¢ņ“µČėµÄČČæÕĘų£¬»įøÉČÅ(5)ÖŠ½įĀŪµÄµĆ³ö£¬ÄćČĻĪŖŹĒ·ńøÉČÅ£¬ĄķÓÉŹĒ________________________________________________________________________”£
¢ŚŹµŃé²Ł×÷¢ó£¬æŖŹ¼Ź±ŃÕÉ«ĪŽĆ÷ĻŌ±ä»ÆµÄŌŅņŹĒ(Š“³öŅ»Ģõ¼“æÉ)________________________________________________________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
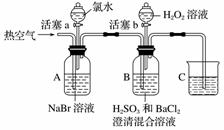
ÓƶžŃõ»ÆĀČ(ClO2)æÉÖʱøÓĆĶ¾¹ć·ŗµÄŃĒĀČĖįÄĘ(NaClO2)£¬ŹµŃéŹŅæÉÓĆĻĀĮŠ×°ÖĆ(ĀŌČ„²æ·Ö¼Š³ÖŅĒĘ÷)ÖʱøÉŁĮæµÄŃĒĀČĖįÄĘ”£
×°ÖĆCÖŠ·¢Éś·“Ó¦£ŗ2NaClO3£«SO2===2ClO2£«Na2SO4(¢ń)
×°ÖĆDÖŠ·¢Éś·“Ó¦£ŗ2ClO2£«H2O2£«2NaOH===2NaClO2£«2H2O£«O2(¢ņ)
(1)ŅĒĘ÷×é×°Ķź±Ļ£¬¹Ų±ÕĮ½øöµÆ»É¼Š£¬“ņæŖAÖŠ»īČū£¬ĻņA֊עČėĖ®æɼģŃé×°ÖĆĘųĆÜŠŌ£¬×°ÖĆĘųĆÜŠŌĮ¼ŗƵÄĻÖĻóŹĒ________________________”£ŹµŃéæŖŹ¼Ź±£¬“ņæŖAµÄ»īČū£¬Į½øöµÆ»É¼ŠµÄæŖ¹Ų²Ł×÷ŹĒ__________________£¬FÖŠŹ¢ÓŠµÄŅŗĢåĪŖ________”£
(2)×°ÖĆBÖŠ½ųŠŠµÄŹĒÓĆĮņĖįÓėŃĒĮņĖįÄĘÖʱø¶žŃõ»ÆĮņµÄ·“Ó¦£¬øĆ“¦Ź¹ÓƵďĒ70%”«80%µÄĮņĖį£¬¶ų²»ŹĒ98%µÄÅØĮņĖį»ņ¼«Ļ”µÄĮņĖį£¬ŌŅņŹĒ____________”£
(3)×°ÖĆCµÄ×÷ÓĆŹĒ________________£¬×°ÖĆEµÄ×÷ÓĆŹĒ________________”£
(4)ŌŚ¼īŠŌČÜŅŗÖŠNaClO2±Č½ĻĪČ¶Ø£¬ĖłŅŌ×°ÖĆDÖŠÓ¦Ī¬³ÖNaOHÉŌ¹żĮ棬Ŋ¶ĻNaOHŹĒ·ń¹żĮæĖłŠčŅŖµÄŹŌ¼ĮŹĒ________”£
a£®Ļ”ŃĪĖį b£®Ę·ŗģČÜŅŗ c£®ŹÆČļČÜŅŗ d£®·ÓĢŖČÜŅŗ
(5)Ņ»“ĪŹµŃéÖʱøÖŠ£¬Ķعż¼ģŃé·¢ĻÖÖʱøµÄNaClO2ÖŠŗ¬ÓŠNaOH”¢Na2SO3£¬³öĻÖÕāŠ©ŌÓÖŹµÄæÉÄÜŌŅņŹĒ__________________”£¼ģŃé²śĪļÖŠŗ¬ÓŠNa2SO3ŌÓÖŹµÄŹµŃé²Ł×÷ŗĶĻÖĻóŹĒ________________________________________________________________________”£
(¹©Ń”ŌńµÄŹŌ¼ĮÓŠ£ŗBa(OH)2ČÜŅŗ”¢H2O2ČÜŅŗ”¢AgNO3ČÜŅŗ”¢Ę·ŗģČÜŅŗ”¢H2SO4ČÜŅŗ)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŠA”¢B”¢C”¢D”¢E”¢FĮłÖÖŌŖĖŲ£¬ŅŃÖŖ£ŗ
¢ŁĖüĆĒĪ»ÓŚČżøö²»Ķ¬¶ĢÖÜĘŚ£¬ŗĖµēŗÉŹżŅĄ“ĪŌö“ó”£
¢ŚEŌŖĖŲµÄµēĄėÄÜŹż¾Ż¼ūĻĀ±ķ(kJ”¤mol£1)£ŗ
| I1 | I2 | I3 | I4 | ” |
| 496 | 4 562 | 6 912 | 9 540 | ” |
¢ŪBÓėFĶ¬Ö÷×唣
¢ÜA”¢E·Ö±š¶¼ÄÜÓėD°“Ō×ÓøöŹż±Č1”Ć1»ņ2”Ć1ŠĪ³É»ÆŗĻĪļ”£
¢ŻB”¢C·Ö±š¶¼ÄÜÓėD°“Ō×ÓøöŹż±Č1”Ć1»ņ1”Ć2ŠĪ³É»ÆŗĻĪļ”£
(1)Š“³öÖ»ŗ¬ÓŠA”¢B”¢D”¢EĖÄÖÖŌŖĖŲµÄĮ½ÖÖĪŽĖ®ŃĪµÄ»ÆѧŹ½______________”¢______________”£
(2)B2A2·Ö×ÓÖŠ“ęŌŚ_________øö¦Ņ¼ü£¬_________øö¦Š¼ü”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖÓŠŅ»·ŻCuOŗĶCu2OµÄ»ģŗĻĪļ£¬ÓĆH2»¹Ō·Ø²ā¶ØĘäÖŠµÄCuOÖŹĮæx g£¬ŹµŃéÖŠæÉŅŌ²ā¶ØŅŌĻĀŹż¾Ż£ŗ¢ŁW£ŗ»ģŗĻĪļµÄÖŹĮæ(g)”¢¢ŚW(H2O)£ŗÉś³ÉĖ®µÄÖŹĮæ(g)”¢¢ŪW(Cu)£ŗÉś³É CuµÄÖŹĮæ(g)”¢¢ÜV(H2)£ŗ±ź×¼×“æöĻĀĻūŗÄH2µÄĢå»ż(L)”£
(ŅŃÖŖĦ¶ūÖŹĮæ£ŗCu£ŗ64 g·mol£1”¢CuO£ŗ80 g·mol£1”¢Cu2O£ŗ144 g·mol£1”¢H2O£ŗ18 g·mol£1)
(1)ĪŖĮĖ¼ĘĖćxÖĮÉŁŠčŅŖ²ā¶ØÉĻŹö4øöŹż¾ŻÖŠµÄ____øö£¬Õā¼øøöŹż¾ŻµÄ×éŗĻ¹²ÓŠ________ÖÖ”£Ēė½«ÕāŠ©×éŗĻŅ»Ņ»ĢīČėĻĀĮŠæÕøńÖŠ”£
ĖµĆ÷£ŗ¢ŁŃ”ÓĆW”¢W(H2O)”¢W(Cu)”¢V(H2)±ķŹ¾£¬²»±ŲĮŠ³ö¾ßĢåĖćŹ½”£
¢ŚĆæøöæÕøńÖŠĢīŅ»ÖÖ×éŗĻ£¬ÓŠ¼øÖÖ×éŗĻ¾ĶĢī¼øÖÖ£¬²»±ŲĢīĀś”£
(2)“ÓÉĻŹö×éŗĻÖŠŃ”³öŅ»øöŗ¬WŹż¾ŻµÄĒóxµÄ¼ĘĖćŹ½£ŗ
________________________________________________________________________ӣ
(3)ŅŌÉĻŹż¾Ż×éŗĻÖŠŅ×ÓŚČ”µĆµÄŅ»×éŹĒ___________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼ĪŖÖ±Į÷µēŌ“µē½āĻ”Na2SO4Ė®ČÜŅŗµÄ×°ÖĆ”£ĶصēŗóŌŚŹÆÄ«µē¼«aŗĶbø½½ü·Ö±šµĪ¼Ó¼øµĪŹÆČļČÜŅŗ”£ĻĀĮŠŹµŃéĻÖĻóĆčŹöÕżČ·µÄŹĒ(””””)

A£®ŅŻ³öĘųĢåµÄĢå»ż£¬aµē¼«µÄŠ”ÓŚbµē¼«µÄ
B£®Ņ»µē¼«ŅŻ³öĪŽĪ¶ĘųĢ壬ĮķŅ»µē¼«ŅŻ³ö“Ģ¼¤ŠŌĘųĢå
C£®aµē¼«ø½½ü³ŹŗģÉ«£¬bµē¼«ø½½ü³ŹĄ¶É«
D£®aµē¼«ø½½ü³ŹĄ¶É«£¬bµē¼«ø½½ü³ŹŗģÉ«
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹¤ŅµÉĻÓƵē½ā·Ø“¦Ąķŗ¬ÄųĖįŠŌ·ĻĖ®²¢µĆµ½µ„ÖŹNiµÄŌĄķČēĶ¼ĖłŹ¾”£
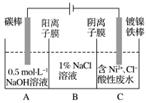
ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ(””””)
ŅŃÖŖ£ŗ¢ŁNi2£«ŌŚČõĖįŠŌČÜŅŗÖŠ·¢ÉśĖ®½ā
¢ŚŃõ»ÆŠŌ£ŗNi2£«(øßÅضČ)£¾H£«£¾Ni2£«(µĶÅضČ)
A£®Ģ¼°ōÉĻ·¢ÉśµÄµē¼«·“Ó¦£ŗ4OH££4e£===O2”ü£«2H2O
B£®µē½ā¹ż³ĢÖŠ£¬BÖŠNaClČÜŅŗµÄĪļÖŹµÄĮæÅØ¶Č½«²»¶Ļ¼õÉŁ
C£®ĪŖĮĖĢįøßNiµÄ²śĀŹ£¬µē½ā¹ż³ĢÖŠŠčŅŖæŲÖĘ·ĻĖ®pH
D£®Čō½«Ķ¼ÖŠŃōĄė×ÓĤȄµō£¬½«A”¢BĮ½ŹŅŗĻ²¢£¬Ōņµē½ā·“Ó¦×Ü·½³ĢŹ½·¢Éśøıä
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«ÉÕ¼īĪüŹÕH2SŗóµÄČÜŅŗ¼ÓČėČēĶ¼ĖłŹ¾µÄµē½ā³ŲµÄŃō¼«Ēų½ųŠŠµē½ā”£µē½ā¹ż³ĢÖŠŃō¼«Ēų·¢ÉśČēĻĀ·“Ó¦£ŗ

S2££2e£===S””(n£1)S£«S2£===S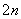
¢ŁŠ“³öµē½āŹ±Ņõ¼«µÄµē¼«·“Ó¦Ź½£ŗ___________________________________”£
¢Śµē½āŗóŃō¼«ĒųµÄČÜŅŗÓĆĻ”ĮņĖįĖį»ÆµĆµ½Įņµ„ÖŹ£¬ĘäĄė×Ó·½³ĢŹ½æÉŠ“³É________________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ķ¬Ņ»·“Ó¦£¬·“Ó¦ĪļדĢ¬²»Ķ¬Ź±
S(g)£«O2(g)===SO2(g)””¦¤H1<0
S(s)£«O2(g)===SO2(g)””¦¤H2<0
Ōņ¦¤H1____¦¤H2”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com