
���������ַ��������Ƶð�ɫ��Fe��OH��2������
 ��������һ���ò���Fe3����FeSO4��Һ���ò���O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
��������һ���ò���Fe3����FeSO4��Һ���ò���O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
������1������������������������FeSO4��Һʱ�������________��
������2����ȥ����ˮ���ܽ��O2������________�ķ�����
������3�����ɰ�ɫFe��OH��2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ������������������_______��
����������������ͼ3��2װ���У���NaOH��Һ����м��ϡNaSO4���Լ��Ʊ���
������1�����Թ�I�������Լ���________��
������2�����Թܢ��������Լ���________��
������3��Ϊ���Ƶð�ɫFe��OH��2���������Թܹ��͢��м����Լ�����ֹˮ�У��������Ӻ��ʵ�鲽����________��
��4���������ɵ�Fe��OH��2�����ܽϳ�ʱ�䱣�ְ�ɫ����������______________��
����һ����1��ϡH2SO4����м��2����У�3���������ɵ�Fe��OH��2�����Ӵ�O2
��������������1��ϡH2SO4����м��2��NaOH��Һ��3�������Թܢ���ڴ��ų��������Ĵ��ȡ����ų���H2����ʱ���ټн�ֹˮ�У�4���Թܢ��з�Ӧ���ɵ�H2�������Թ�I���Թܢ�����������������
����������һ�и���FeSO4�����ױ���������ˮ����ص㣬����������Һʱ�������ϡH2SO4����м������ˮ��������O2��������Fe��OH��2��������˿ɲ�����з���ȥO2��Ϊ�������ɵ�Fe��OH��2�����Ӵ�O2�����ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ���ɴﵽĿ�ġ�
������������ԭ��������ϡH2SO4����м�����������ȸϾ�װ���п�����ȷ���Ͼ��������ټн�ֹˮ�У�ͨ��H2������ѹǿ��FeSO4ѹ��NAOH��Һ��ȥ���Ӷ�ʹ�����İ�ɫFe��OH��2������H2�Ļ�ԭ�������е��Խϳ�ʱ�䱣�֡�
����������һ����Fe��OH��2���Ʊ�ԭ���йص���Ŀ�������˴��µ�ʵ���ַ����Դ�����Ŀ��������������ڶԻ���֪ʶ����ʵ���գ����й�ʵ��ԭ������������֪�̶ȣ�����Ҫ�Ŀ��غʹ��¾���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 33�����������ַ��������Ƶð�ɫ��Fe��OH��2������
33�����������ַ��������Ƶð�ɫ��Fe��OH��2�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?��ɽ��ģ�����������ַ��������Ƶð�ɫ��Fe��OH��2������
��2011?��ɽ��ģ�����������ַ��������Ƶð�ɫ��Fe��OH��2�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ���ò���Fe3+��FeSO4��Һ���ò���O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
��1������������������������FeSO4��Һʱ������___________________________________��
��2����ȥ����ˮ���ܽ��O2������______________�ķ�����
��3�����ɰ�ɫFe(OH)2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ������������������______________________________________��
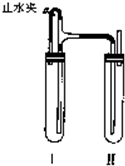
������������ͼװ���У���NaOH��Һ����м��ϡH2SO4���Լ��Ʊ���

��1�����Թܢ��������Լ���____________________________________��
��2�����Թܢ��������Լ���____________________________________��
��3��Ϊ���Ƶð�ɫFe(OH)2���������Թܢ�͢��м����Լ�����ֹˮ�У��������Ӻ��ʵ�鲽����___________________________________________________________________��
��4���������ɵ�Fe(OH)2�����ܽϳ�ʱ�䱣�ְ�ɫ����������_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ַ��������Ƶð�ɫ��Fe(OH)2����������һ���ò���Fe3+��FeSO4��Һ���ò���O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ�����1������������������������FeSO4��Һʱ�������________________________________��
��2����ȥ����ˮ���ܽ��O2�����õķ�����_______________________________________��
��3�����ɰ�ɫFe(OH)2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ������������������_____________________________________
_______________________________________________________________________________��
��������������ͼװ���У���NaOH��Һ����м��ϡH2SO4���Լ��Ʊ���
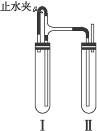
��1�����Թܢ��������Լ���___________________��
��2�����Թܢ��������Լ���___________________��
��3��Ϊ���Ƶð�ɫFe(OH)2���������Թܢ�͢��м����Լ�����ֹˮ�У��������Ӻ��ʵ�鲽����_______________________________________________________________________��
��4���������ɵ�Fe(OH)2�����ܽϳ�ʱ�䱣�ְ�ɫ����������________________________
_______________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ������ѧ�ڵڶ����¿���ѧ�Ծ��������棩 ���ͣ������
��12�֣����������ַ��������Ƶð�ɫ��Fe(OH)2������
����һ���ò���Fe3����FeSO4��Һ�벻��O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
������������������������FeSO4��Һʱ�������____________��
�Ƴ�ȥ����ˮ���ܽ��O2������________�ķ�����
�����ɰ�ɫFe(OH)2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ������������������______________________________��

������������ͼ��ʾװ���У���NaOH��Һ����м��ϡH2SO4���Լ��Ʊ���
�����Թܢ��������Լ���____________��
�����Թܢ��������Լ���____________��
���������ɵ�Fe(OH)2�����ܽϳ�ʱ�䱣�ְ�ɫ����������____________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com