

| �����Ӧ�� | a | b | c | d |
| n��SO32-����n��HSO32-�� | / | 91��9 | 1��1 | 9��91 |
| pH | ��8.2 | 8.2 | 7.2 | 6.2 |
| A�� | b����Һ�У�ˮ�������c��OH-��=1��10-5.8mol/L | |
| B�� | c����Һ�У�c��Na+����c��SO32-����c��HSO3-����c��OH-����c��H+�� | |
| C�� | d����Һ��HSO3-�ĵ������ˮ�� | |
| D�� | d����Һ�У�c��Na+����c��SO32-��+c��HSO3-��+c��Cl-�� |
���� a����ҺPH��8.2����Һ�Լ��ԣ�������������Һ�������������ˮ���ԭ����1L0.1mol/L��Na2SO3��Һ�еμ����ᣨ���跴Ӧǰ����Һ������䣩����Һ��ˮ�������OH-����Ũ���������������ϵ���ɱ����е����ݿ�֪��HSO3-Խ�࣬����Խǿ����������������ӣ�����Һ������ʱ������Ϊ�������ƺ����������ƣ�������ˮ��ij̶���ȣ�
A��b����Һ�д������ӻ���������ҺPH=8.2��c��H+��=10-8.2mol/L��ˮ�������c��OH-��=$\frac{{K}_{w}}{c��{H}^{+}��}$��
B��c����ҺPH=7.2��n��SO32-��n����HSO3-��=1��1����Һ�Լ���˵��������������ӵ�����������������ˮ�⣬��Һ��c��OH-����c��H+����c��SO32-����c��HSO3-����
C��d����ҺPH=6.2��Һ�����ԣ�n��SO32-��n����HSO3-��=9��91��������Һ��ΪNaHSO3��NaCl��ҺPH��7��˵��HSO3-�ĵ������ˮ�⣻
D��d����Һ�д��ڵ���غ㣬��Һ������c��OH-����c��H+����
��� �⣺A��b����Һ�д������ӻ���������ҺPH=8.2��c��H+��=10-8.2mol/L��ˮ�������c��OH-��=$\frac{{K}_{w}}{c��{H}^{+}��}$=$\frac{1{0}^{-14}}{1{0}^{-8.2}}$mol/L=1��10-5.8mol/L����A��ȷ��
B��c����ҺPH=7.2��n��SO32-��n����HSO3-��=1��1����Һ�Լ���˵��������������ӵ�����������������ˮ�⣬��Һ��c��OH-����c��H+����c��SO32-����c��HSO3-������Һ������Ũ��c��Na+����c��SO32-����c��HSO3-����c��OH-����c��H+������B��ȷ��
C��d����ҺPH=6.2��Һ�����ԣ�n��SO32-��n����HSO3-��=9��91��������Һ��ΪNaHSO3��Na2SO3��NaCl����ҺPH��7��˵��HSO3-�ĵ������ˮ�⣬��C��ȷ��
D��d����Һ�д��ڵ���غ㣬c��Na+��+c��H+��=2c��SO32-��+c��HSO3-��+c��OH-��+c��Cl-����c��H+����c��OH-������Һ������c��Na+����2c��SO32-��+c��HSO3-��+c��Cl-������D����
��ѡD��
���� ���⿼����ͼ�������������ʵ���ƽ�⣬����ˮ��ƽ�⣬�������Һ������Ũ�ȴ�С�Ƚϣ���Һ�е���غ㡢�����غ��֪ʶ��Ӧ�ã���ȷͼ��仯�����ǽⱾ��ؼ�����Ŀ�Ѷ��еȣ�


 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
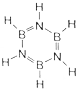 ��Ԫ����һ�ֳ���Ԫ�أ����γɶ��ֻ����
��Ԫ����һ�ֳ���Ԫ�أ����γɶ��ֻ����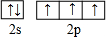 �������ʵ�����NH3��N2��ɵĻ�������ЦҼ��ͦм�������֮��Ϊ2��1��
�������ʵ�����NH3��N2��ɵĻ�������ЦҼ��ͦм�������֮��Ϊ2��1��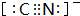 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Zn��������Ag2O�Ǹ��� | |
| B�� | ʹ�ù����У�������Ag2O������·����Zn�� | |
| C�� | ʹ�ù����У��缫��������Һ��pH��С | |
| D�� | Zn�缫����������Ӧ��Ag2O�缫������ԭ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ������ұ��������4�֣��������ȷ�Ӧ�����ȷֽⷨ | |
| B�� | �����е�Ԫ�صļ���ԭ����2H++2I-+H2O2=I2+2H2O | |
| C�� | ʯ�͵ķ����ѻ����ѽ⣬���������ѻ��������仯 | |
| D�� | ���ȷ�Ӧ��������ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
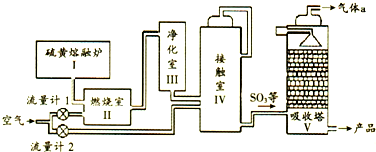
| A�� | ������Ҫ��Ӧ���Ȼ�ѧ����ʽ��S��s��+O2��g��?SO3��g����H=-297kJ•mol-1S��s��+O2��s��=SO2��g����H=-297kJ•mol-1 | |
| B�� | ������Ҫ��Ӧ�Ļ�ѧ����ʽ��2SO2+O2��s�� $?_{��}^{����}$SO3 | |
| C�� | ����ʹ�ô��������ѧ��Ӧ���ʺ�ƽ��ת���� | |
| D�� | ��������a ����ֱ���ŷŵ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ����Ԫ�����ڱ��е�λ����ͼ������ֻ��һ�ֲ��Ƕ�����Ԫ�أ�ֻ��һ���ǽ���Ԫ�أ�����˵����ȷ���ǣ�������
����Ԫ�����ڱ��е�λ����ͼ������ֻ��һ�ֲ��Ƕ�����Ԫ�أ�ֻ��һ���ǽ���Ԫ�أ�����˵����ȷ���ǣ�������| A�� | �����Ӱ뾶��С��Q��Y��X | |
| B�� | �����̬�⻯��ķе�ߵͣ�Y��Z | |
| C�� | Q��������������X���⻯��ֱ�ӷ�Ӧ | |
| D�� | X��Y�ȿɴ�����ͬһ���ۻ������У�Ҳ�ɴ�����ͬһ���ӻ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CaCl2�мȺ������Ӽ��ֺ��й��ۼ� | |
| B�� | Na0H�мȺ������Ӽ��ֺ��й��ۼ� | |
| C�� | Na2O2��ֻ�������Ӽ� | |
| D�� | �ɷǽ���Ԫ����ɵ�����NH4Cl��ֻ�й��ۼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������ı��ӿ��ȼ�������Ũ��ˮ��ʹ֮�������屽�ӣ��ٹ��˳�ȥ | |
| B�� | �����Ӿ����������ˮ�У�����ʱȫ���ܽ⣬��ȴ����Һ�Գ����� | |
| C�� | ���ӵ����Ժ���������ʹָʾ����ɫ����������NaCHO3��Ӧ�ų�CO2 | |
| D�� | �����ж�������ϡ��Һ��ֱ�������������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���ܶ�����л�����E���¿���H2�����ӳɷ�Ӧ�����CH2=CH2����HC��CH���۱�����HCHO������̼ԭ�Ӳ�ȡsp2�ӻ��ķ����Т٢ۢܣ���������ţ���HCHO���ӵ�����ṹΪƽ�������Σ����ӳɲ�����ۡ��е��CH4���ۡ��е�ߣ�����Ҫԭ���ǣ���ָ���ӳɲ����Ǻ����ʣ��ӳɲ���CH3OH����֮�����γ������
���ܶ�����л�����E���¿���H2�����ӳɷ�Ӧ�����CH2=CH2����HC��CH���۱�����HCHO������̼ԭ�Ӳ�ȡsp2�ӻ��ķ����Т٢ۢܣ���������ţ���HCHO���ӵ�����ṹΪƽ�������Σ����ӳɲ�����ۡ��е��CH4���ۡ��е�ߣ�����Ҫԭ���ǣ���ָ���ӳɲ����Ǻ����ʣ��ӳɲ���CH3OH����֮�����γ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com