
| 1 |
| 5 |
| ||


 С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2009?�Ϻ������ݱ��ṩ�����ݣ��ж��ڵ�Ũ�ȵ�NaClO��NaHCO3�����Һ�У���������Ũ�ȹ�ϵ��ȷ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�ر�� | a | b | c | d | e | f | g | h | i | j |
| ԭ�Ӱ뾶/pm | 111 | 77 | 70 | 104 | 143 | 99 | 117 | 186 | 160 | 64 |
| ����ϼۻ� ��ͻ��ϼ� |
+2 | -4 | -3 | +6 | +3 | -1 | +4 | +1 | +2 | -1 |
| ���� ���� |
��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 1 | ||||||||
| 2 | a a |
b b |
c c |
j j |
||||
| 3 | h h |
i i |
e e |
g g |
d d |
f f |
||
| ||
| ||



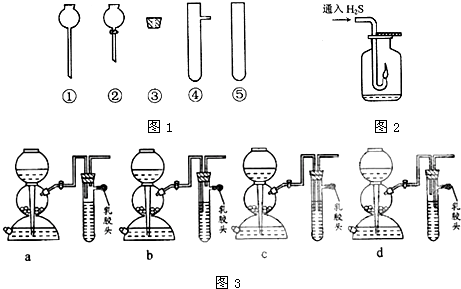
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com