
���� ��1���������ɵ��ӡ��������ӵĿɵ��磬��ˮ��Һ�л��ۻ�״̬�µ���Ļ�����Ϊ����ʣ�
��2���ۺ͢���Һ��Ӧ�������ᱵ��ˮ��
��3��A������ʱ�������ᱵ�������ƺ�ˮ��
B����SO42-ǡ����ȫ��������1��1��Ӧ���������ᱵ��NaOH��ˮ��
C������Ba��OH��2��Һ���������������ᱵ��NaOH��ˮ��
��� �⣺�������������ɵ��ӣ�Ϊ���ʣ���K2OΪ���ӻ������Ba��OH��2•8H2OΪ���ӻ���������ᱵΪ���ӻ�����ݴ�����Ϊ���ۻ������Һ̬�Ĵ���Ϊ�������ʯ��ˮΪ���������ɵ��ӣ����Ҵ�Ϊ�ǵ���ʣ����ۻ�������غ������ƶ������ӣ�Ϊ���ӻ����
��1�������ܵ�����Ǣ٢ߢᣬ���ڵ���ʵ��Ǣڢۢܢݢޢᣬ�ʴ�Ϊ���٢ߢ�ڢۢܢݢޢ
��2���ۺ͢���Һ��Ϻ�����Ӧ�����ӷ���ʽΪ2OH-+2H++SO42-+Ba2+=BaSO4��+2H2O���ʴ�Ϊ��2OH-+2H++SO42-+Ba2+=BaSO4��+2H2O��
��3��A������ʱ�������ᱵ�������ƺ�ˮ�����ӷ�ӦΪ2OH-+2H++SO42-+Ba2+=BaSO4��+2H2O����ѡ��
B����SO42-ǡ����ȫ��������1��1��Ӧ���������ᱵ��NaOH��ˮ�����ӷ�ӦΪOH-+H++SO42-+Ba2+=BaSO4��+H2O���ʲ�ѡ��
C������Ba��OH��2��Һ���������������ᱵ��NaOH��ˮ�����ӷ�ӦΪOH-+H++SO42-+Ba2+=BaSO4��+H2O���ʲ�ѡ��
�ʴ�Ϊ��A��
���� ���⿼�����ӷ�Ӧ����ʽ����д��Ϊ��Ƶ���㣬���յ���ʡ����ӷ�Ӧ��Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬�ۺ��Խ�ǿ����Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaHCO3����Һ����NaHCO3�TNa++H++CO32- | |
| B�� | NaHSO4����Һ����NaHSO4�TNa++H++SO42- | |
| C�� | ��NH4��2SO4����Һ������NH4��2SO4�T��NH4��2++SO42- | |
| D�� | CuCl2����Һ����CuCl2�TCu2++Cl2- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���þ۹��ֵ�Ͳ���䣬����������Һ�ͽ��� | |
| B�� | ��Һ�����塢����Һ������Һ�ȷ�ɢϵ�е����ʾ��Է�����ʽ���� | |
| C�� | ��ɢ�����ӵ�ֱ����1��10-9��1��10-7m֮��ķ�ɢϵ���ڽ��� | |
| D�� | ����ˮ������ijЩ���λ���������ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
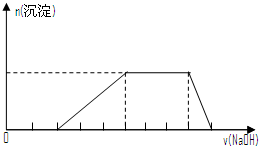
| A�� | ��NaHCO3��Na2CO3�����6.85g����ˮ�Ƴ�100mL��Һ������c��Na+��=1mol•L-1������Һ�м���һ��������ǡ����ȫ��Ӧ������Һ���ɺ����ù������������� | |
| B�� | ��54.4g���ۺ��������Ļ�����м���4.0mol/L 200mL��ϡ���ᣬǡ����ȫ��Ӧ���ų�����4.48L����״��������Ӧ�����Һ�еμ�KSCN���Ժ�ɫ��������ʣ�����Ӧ��õ�FeSO4�����ʵ�����0.8mol | |
| C�� | ����һ�ܱ������г���1molN2��3molH2����һ��������ʹ�÷�Ӧ�������ﵽ��ѧƽ��ʱ��N2��H2��NH3�����ʵ���Ũ��һ����� | |
| D�� | ij��Һ�п��ܺ���H+��Na+��NH${\;}_{4}^{+}$��Mg2+��Fe3+��Al3+��SO${\;}_{4}^{2-}$�����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ��ʾ���ɴ˿�֪������Һ�п϶����е���������H+��NH${\;}_{4}^{+}$��Mg2+��Al3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
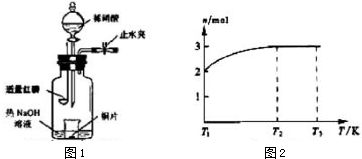
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ȼ����ú��ʯ�Ͷ��������Դ | |
| B�� | ����ú���Եõ����顢���Ͱ�����Ҫ����ԭ�� | |
| C�� | ú�ĸ����������仯��ú��������Һ���ǻ�ѧ�仯 | |
| D�� | Һ��ʯ��������Ȼ������Ҫ�ɷֶ��Ǽ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 4 | B�� | 5 | C�� | 6 | D�� | 7 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com