
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ��������� ��
��2���ձ���������ֽ���������� ��
��3��Ҫ�ظ���������ʵ���Ŀ���� ��
��4�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ (�ƫ��ƫС����Ӱ�족)�������µ���10��ʱ���У���ʵ��������ɽϴ������ԭ����_______ ��
��5�������60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� (���ȡ�����ȡ�)�������к��� (���ȡ�����ȡ�)���������� ��
��6������ͬŨ�Ⱥ�����Ĵ��ᣨCH3COOH������HCl��Һ��������ʵ�飬��õ��к��ȵ���ֵ�� �����ƫ��ƫС����Ӱ�족����
��7������ƽ�в�������õ��������£�
| �¶� ��� | ��ʼ�¶�t1/�� | ��ֹ�¶� T2/�� | �¶Ȳ� ��t/�� | ||
| HCl | NaOH | ƽ��ֵ | |||
| 1 | 25 | 25 | | 27.3 | |
| 2 | 25 | 25 | | 27.4 | |
| 3 | 25 | 25 | | 28.6 | |
��1������������ (2��)
��2�����ȱ��¡�����ʵ���������������ʧ ��2�֣�
��3����β�����ƽ��ֵ���Լ���ʵ����� ��2�֣�
��4��ƫС��1�֣������½ϵ�ʱ��Ӧ��ϵ��ɢ�ȱȽϿ죬������ʧ���ƫ�ͣ�1�֣�
��5������ȣ�1�֣�����ȣ�1�֣����к�����ָ�������кͷ�Ӧ����1molH2Oʱ���ų������������ᡢ��������أ�2�֣�
��6��ƫС��1�֣�
��7��39.3kJ/mol ��H= -39.3kJ/mol�� ��2�֣�
����


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
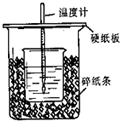 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1�����ⶨ��20g������������ȼ������ˮ����������2418.0kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ
��1�����ⶨ��20g������������ȼ������ˮ����������2418.0kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��50mL0.50mol?L-1������50mL0.55mol?L-1 NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol?L-1������50mL0.55mol?L-1 NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
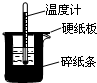 50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com