
��.ʵ�������CuO��H2��ԭʱҲ��Cu2O���ɡ���һ������H2����ͨ�����ȵ�CuO��ĩ���õ����������һ������m(Cu)��m(O)=8��a����a�в�ͬ��ȡֵʱ����������ɷֲ�ͬ�������a��ȡֵ��Χ��������ɷֵĹ�ϵ�������±�����һ������������Ҳ���������ӣ���
a��ȡֵ��Χ | ��Ӧ�����ijɷ֣��û�ѧʽ��ʾ�� |
|
|
|
|
|
|
��.��ͭ�����Ҫ�ɷ�X����Cu��Fe��S����Ԫ����ɵĸ��Σ�����Cu��Fe����Ԫ�ص�������Ϊ8��7����m g X��ĩȫ������200 mL��ŨHNO3����Ӧ�����Һ��ˮϡ����2.12 Lʱ�����pHΪ0����ϡ�ͺ����Һ��Ϊ���ȷݣ�������һ����Һ�еμ�6.05 mol��L-1��NaOH��Һ������һ����Һ�еμ�0.600 mol��L-1 Ba(NO3)2��Һ������Һ�о����ɳ������ҳ�����������������Һ������仯����ͼ��ʾ��
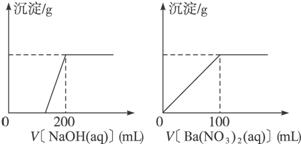
��1����ͨ������ȷ��m��ֵ��
��2��X��Ħ������Ϊ368 g��mol-1,��ȷ��X�Ļ�ѧʽ_______________
��.
A��ȡֵ��Χ | ��Ӧ���������ijɷ֣��û�ѧʽ��ʾ�� |
1��a��2 | CuO��Cu2O |
0��a��1 | Cu2O��Cu |
��.(1)m=11.04 (2)Cu2Fe2S4
��������.��m(Cu)��m(O)=64��16=8��2ʱ��a=2,ȫ����CuO;
��m(Cu)��m(O)=64��8=8��1ʱ,a=1,����ȫ����Cu2O�� ��ʱ1��a��2
��m(Cu)��m(O)=64��0,��ȫ��ΪCuʱ,a=0��ʱ0��a��1��
��.��1�������⣬m g X�С�n(Cu)��n(Fe)=1��1
2n(Cu2+)+3n(Fe3+)=6.05 mol��L-1��0.2 L��2-1 mol��L-1��2.12 L
�ʣ�n(Cu)=n(Fe)=0.06 mol
�֣�n(S)=0.6 mol��L-1��0.1 L��2=0.12 mol
��ˣ�m g=m(Cu)+m(Fe)+m(S)=0.06 mol��64 g��mol-1+0.06 mol��56 g��mol-1+0.12 mol��32 g��mol-1=11.04 g
��m��ֵΪ11.04
(2)��X�Ļ�ѧʽΪ��CuFeS2��n,��
��64+56+32��2����n=368 n=2
��X�Ļ�ѧʽΪCu2Fe2S4��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ͭ����������ʹ�õĽ��������㷺Ӧ���ڵ������Ṥ����е���������ҵ�ȣ���֪Cu2O��H2SO4�ܷ�����Ӧ��Cu2O+H2SO4=Cu+CuSO4+H2O��
ͭ����������ʹ�õĽ��������㷺Ӧ���ڵ������Ṥ����е���������ҵ�ȣ���֪Cu2O��H2SO4�ܷ�����Ӧ��Cu2O+H2SO4=Cu+CuSO4+H2O��
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��.ʵ�������CuO��H2��ԭʱҲ��Cu2O���ɡ���һ������H2����ͨ�����ȵ�CuO��ĩ���õ����������һ������m(Cu)��m(O)=8��a����a�в�ͬ��ȡֵʱ����������ɷֲ�ͬ�������a��ȡֵ��Χ��������ɷֵĹ�ϵ�������±�(��һ������������Ҳ����������)��
a��ȡֵ��Χ | ��Ӧ�����ijɷ�(�û�ѧʽ��ʾ) |
|
|
|
|
|
|
��.��ͭ�����Ҫ�ɷ�X����Cu��Fe��S����Ԫ����ɵĸ��Σ�����Cu��Fe����Ԫ�ص�������Ϊ8��7����m g X��ĩȫ������200 mL��ŨHNO3����Ӧ�����Һ��ˮϡ����2.12 Lʱ�����pHΪ0����ϡ�ͺ����Һ��Ϊ���ȷݣ�������һ����Һ�еμ�6.05 mol��L-1��NaOH��Һ������һ����Һ�еμ�0.600 mol��L-1 Ba(NO3)2��Һ������Һ�о����ɳ������ҳ�����������������Һ������仯����ͼ��ʾ��
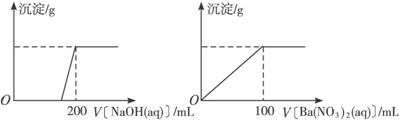
(1)��ͨ������ȷ��mֵ��
(2)X��Ħ������Ϊ368 g��mol-1����ȷ��X�Ļ�ѧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ������һ�и���9���¿������ۺϻ�ѧ�Ծ����������� ���ͣ�������
��16�֣�. ͭ����������֪���Ľ���֮һ��ʵ���ҿ���C��H2��ԭCuO��ȡ������Cu����ҵ����Ҫ�ûӻ�ͭ��������Cu��
��. ʵ��֤����C��ԭCuOʱ�ȿ�������Cu��Ҳ��������Cu2O����ʹ��C����ʱ��ʵ����CuOҲ���ܲ���δ����ԭ��Ϊ�˲ⶨij��ʵ��������ɣ�ȡ1.2 g C��8.0 g CuO��ϼ��ȣ�����Ӧ���ɵ�����ͨ�������ij���ʯ��ˮ��������һ��ʱ���ֹͣ���ȣ����ռ���560 mL���壨�Ѿ�����ɱ�״��������ó���������Ϊ2.5 g����
��1������ʵ����C �����ȫ������ȫ�����μӷ�Ӧ��ʵ�����ռ�������
���� ����д��ѧʽ����������������Ļ�ѧ����ʽΪ�� ��
��2����Ӧ��õ��Ĺ�������������Ϊ �����к�������������ʵ���Ϊ mol��
��. ��ͭ�����Ҫ�ɷ�X����Cu��Fe��S����Ԫ����ɵĸ��Σ�����Cu��Fe����Ԫ�ص�������Ϊ8��7����m g X��ĩȫ������200 mL��ŨHNO3����Ӧ�����Һ��ˮϡ���� 2��12 Lʱ�����pHΪ0����ϡ�ͺ����Һ��Ϊ���ȷݣ�������һ����Һ�еμ�6.05mol/L��NaOH��Һ������һ����Һ�еμ�0��600mol/LBa(NO3)2��Һ������Һ�о����ɳ������ҳ�����������������Һ������仯����ͼ��ʾ��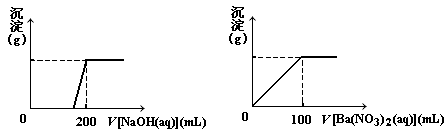
��1�� ��ͨ������ȷ��m��ֵ��
��2�� X��Ħ������Ϊ368 g/mol����ȷ��X�Ļ�ѧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�����и���9���¿������ۺϻ�ѧ�Ծ��������棩 ���ͣ�������
��16�֣�. ͭ����������֪���Ľ���֮һ��ʵ���ҿ���C��H2��ԭCuO��ȡ������Cu����ҵ����Ҫ�ûӻ�ͭ��������Cu��
��. ʵ��֤����C��ԭCuOʱ�ȿ�������Cu��Ҳ��������Cu2O����ʹ��C����ʱ��ʵ����CuOҲ���ܲ���δ����ԭ��Ϊ�˲ⶨij��ʵ��������ɣ�ȡ1.2 g C��8.0 g CuO��ϼ��ȣ�����Ӧ���ɵ�����ͨ�������ij���ʯ��ˮ��������һ��ʱ���ֹͣ���ȣ����ռ���560 mL���壨�Ѿ�����ɱ�״��������ó���������Ϊ2.5 g����
��1������ʵ����C �����ȫ������ȫ�����μӷ�Ӧ��ʵ�����ռ�������
���� ����д��ѧʽ����������������Ļ�ѧ����ʽΪ�� ��
��2�� ��Ӧ��õ��Ĺ�������������Ϊ �����к�������������ʵ���Ϊ mol��
��. ��ͭ�����Ҫ�ɷ�X����Cu��Fe��S����Ԫ����ɵĸ��Σ�����Cu��Fe����Ԫ�ص�������Ϊ8��7����m g X��ĩȫ������200 mL��ŨHNO3����Ӧ�����Һ��ˮϡ���� 2��12 Lʱ�����pHΪ0����ϡ�ͺ����Һ��Ϊ���ȷݣ�������һ����Һ�еμ�6.05mol/L��NaOH��Һ������һ����Һ�еμ�0��600mol/L Ba(NO3)2��Һ������Һ�о����ɳ������ҳ�����������������Һ������仯����ͼ��ʾ��
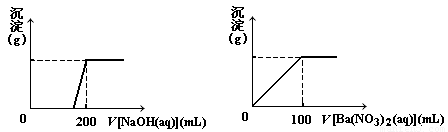
��1�� ��ͨ������ȷ��m��ֵ��
��2�� X��Ħ������Ϊ368 g/mol����ȷ��X�Ļ�ѧʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com