
���� ��1������ʵ������IJ��裨���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����ѡ����Ҫ�����������жϲ���Ҫ��������
��2�����ݣ�1���ж�ȱ�ٵ�������
��3������һ�����ʵ���Ũ����Һ��һ�㲽��Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���ݴ�����
��4���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��5������m=CVM������Ҫ���ʵ�������������Һϡ���������ʵ����ʵ������������ҪŨ��Һ�������
��� �⣺��1��ʵ������IJ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬���ò��������裬�ָ����º�ת�Ƶ�100mL����ƿ�У����ò�����������ϴ���ձ��Ͳ�����2��3�Σ�����ϴ��Һ��������ƿ�У���������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμ�ˮ����Һ����̶���ˮƽ���У��Ǻ�ƿ���������ߵ�����ҡ�ȣ������Լ�ƿ����ǩ���森
������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ιܣ�������ϴƿ��ˮ����ѡCDE��
�ʴ�Ϊ��CDE��
��2�����ݣ�1����֪ȱ�ٵ������У��ձ�����������
�ʴ�Ϊ���ձ�����������
��3������һ�����ʵ���Ũ����Һ��һ�㲽��Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������ȷ��˳��Ϊ��BCAFED��
�ʴ�Ϊ��BCAFED��
��4��A������ʱ��������ƿ�̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���Aѡ��
B������ʱ��������ƿ�̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���B��ѡ��
C���Ȼ����ܽ��������������Խ��ܽ���ȴ�����Һת������ƿ��ͽ��ж��ݲ������ָ������£����ƫ����ҺŨ��ƫ�ͣ���C��ѡ��
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ���������Һ���ƫ����ҺŨ��ƫ�ͣ���D��ѡ��
��ѡ��A��
��5������100mL 0.5mol/L��NaCl��Һ����Ҫ���ʵ�������0.1L��0.5mol/L��58.5g/mol=2.9g������4mol/L��NaClŨ��Һ����100mL 0.5mol/L��ϡ��Һ����������Ͳ��ȡ��Һ���V����������Һϡ���������ʵ����ʵ����������ã�4mol/L��V=100mL��0.5mol/L�����V=12.5mL��
�ʴ�Ϊ��2.9��12.5��
���� ���⿼��һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ�������������ǽ���ؼ���ע���������ķ�������Ŀ�ѶȲ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ĭ���������Al3+��HCO3-���Ӧ�ɷų�������̼���� | |
| B�� | ʹ���ȵĴ���ˮϴ��ʱ�����׳�ȥ���ϵ���֬�۹� | |
| C�� | ���������ھ�ˮ | |
| D�� | ͭ�̵����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | þ����������Ͻ�������ɻ����������Ҫ���� | |
| B�� | �ߴ��ȹ��ƳɵĹ��أ�����������̽�����Ķ��� | |
| C�� | ��֬��ά�����������ǽ������ϣ���������� | |
| D�� | ���½ṹ�մ����¡������������������������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
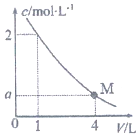 ��ͼ��BaC12��Һ��ϡ�����У�c��Ba2+������Һ����ı仯����ͼ����M��ʱ����Һ��c��Cl-��Ϊ��������
��ͼ��BaC12��Һ��ϡ�����У�c��Ba2+������Һ����ı仯����ͼ����M��ʱ����Һ��c��Cl-��Ϊ��������| A�� | 0.25mol/L | B�� | 0.5mol/L | C�� | 1 mol/L | D�� | 2mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij�������ϡ���ᣬ��������ɫ���壬֤���ù���һ����̼���� | |
| B�� | ij��Һ�еμ�BaCl2��Һ�����ɲ�����ϡHNO3�İ�ɫ����������Һ��һ����SO42- | |
| C�� | ij��ɫ��Һ�е�����ɫ��̪�Ժ�ɫ������Һһ���Լ��� | |
| D�� | ��֤�ռ���Һ���Ƿ���Cl-���ȼ�ϡ�����ȥOH-���ټ�AgNO3��Һ���а�ɫ�������֣�֤����Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
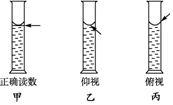 ������һ�����ʵ���Ũ����Һ��ʵ���У�����Ͳȥȡ��һ������Ũ��Һ����Һʱ����Ͳ�����ƽ������Ҫ����Ͳ��Һ��İ�Һ����͵㱣��ˮƽ����ͼ�ף����ٶ���Һ����������
������һ�����ʵ���Ũ����Һ��ʵ���У�����Ͳȥȡ��һ������Ũ��Һ����Һʱ����Ͳ�����ƽ������Ҫ����Ͳ��Һ��İ�Һ����͵㱣��ˮƽ����ͼ�ף����ٶ���Һ�����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ag+��Cu2+��Sb3+ | B�� | Cu2+��Ag+��Sb3+ | C�� | Sb3+��Ag+��Cu2+ | D�� | Ag+��Sb3+��Cu2+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com