
��1��Ԫ��M�Ƕ�����Ԫ�أ��䳣�������ں�ˮ�У����ʱ���Ϊ��������������
��M��ԭ�ӽṹʾ��ͼΪ______��
����M��AlΪ�缫��KOH��ҺΪ�������Һ�����ĵ缫��ӦʽΪ______��
��2������ǽ������������ȵ�ij�¶ȣ��漴�����������п�����ȴ�Ľ����ȴ������ա�
��ʹ��ˮ���д�������ɴ������������÷�Ӧ�Ļ�ѧ����ʽΪ____________
����֤����ˮ����Ĺ�������Ƿ����+3�۵�������ѡ�õ��Լ�Ϊ_______ (�����)
| A��H2O2��Һ | B��ͭ�� | C��ϡ���� | D��KMnO4��Һ |
 4Fe(OH)3+8OH��+3O2
4Fe(OH)3+8OH��+3O2
��15�֣���1����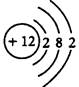 ��2�֣�
��2�֣�
��Al+4OH����3e��=[Al(OH)4]����AlO2��+2H2O��2�֣�
��2����3Fe+4H2O Fe3O4+4H2����3�֣�
Fe3O4+4H2����3�֣�
��BC��2�֣�
��3����6.9��10��7mol?L��1?mol��1��2�֣�
�ڲ��䣨2�֣�
��>��2�֣�
���������������1���ٸ��������֪MΪþ���˵����Ϊ+12��������Ӳ�ṹΪ282����þ������KOH��Һ����������KOH��Һ������������������������Ӧ����ӦʽΪAl+4OH����3e��=[Al(OH)4]����AlO2��+2H2O����2���ٺ��ȵ�������ˮ��Ӧ��������������������������3Fe+4H2O Fe3O4+4H2������Fe3O4�Ǽ��������������ϡ���ᣬ����ˮ��Fe3+��Fe2+��˫��ˮ������ؾ�������Fe2+��ͭ���ܻ�ԭFe3+��Fe3+������ͭ����BC��ȷ����3���ٶ�ͼ��֪����c(FeO42��)=(1.0��0.45)��10��3mol/L���ɡ�c/��t��֪��v(FeO42��)=(1.0��0.45)��10��3mol/L��800min=6.867��10��7mol/(L?min)��6.9��10��7mol/(L?min)��������pH����¶Ȳ��䣬��ƽ�ⳣ�����䣻�������¶����ߣ�c(FeO42��)��С����ƽ�����ƣ�˵������Ӧ�����ȷ�Ӧ�����H>0��
Fe3O4+4H2������Fe3O4�Ǽ��������������ϡ���ᣬ����ˮ��Fe3+��Fe2+��˫��ˮ������ؾ�������Fe2+��ͭ���ܻ�ԭFe3+��Fe3+������ͭ����BC��ȷ����3���ٶ�ͼ��֪����c(FeO42��)=(1.0��0.45)��10��3mol/L���ɡ�c/��t��֪��v(FeO42��)=(1.0��0.45)��10��3mol/L��800min=6.867��10��7mol/(L?min)��6.9��10��7mol/(L?min)��������pH����¶Ȳ��䣬��ƽ�ⳣ�����䣻�������¶����ߣ�c(FeO42��)��С����ƽ�����ƣ�˵������Ӧ�����ȷ�Ӧ�����H>0��
���㣺����þ�Ĵ��ڡ�ԭ�ӽṹʾ��ͼ�����͵�صĸ�����Ӧʽ��������Ҫ���ʡ������仯��������ʡ���ѧ��Ӧ���ʡ���ѧƽ�ⳣ������Ӧ�ȡ��¶ȶԻ�ѧƽ���Ӱ������֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��NaOH��MgCl2��AlCl3���ֹ�����ɵĻ������������ˮ����1.16g��ɫ����������������Һ������1.00mol/LHCl��Һ������HCl��Һ����������ɳ����Ĺ�ϵ��ͼ��ʾ��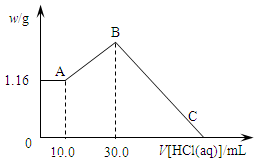
��1��A��ij�����Ļ�ѧʽΪ ��
��2��д��A����B�㷢����Ӧ�����ӷ���ʽ�� ��
��3��ԭ�������NaOH�������� g��C�㣨��ʱ����ǡ����ȫ�ܽ⣩HCl��Һ�����Ϊ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��ϸ��������ˮ��Һ�����������£����Խ���ͭ����Ҫ�ɷ���CuFeS2������������SiO2�������������Ρ����ø�ԭ������ͭ���̷���FeSO4��7H2O�����������£�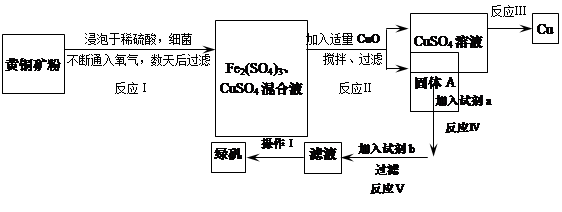
�ش��������⣺
��1����֪��
| | Fe2+ | Cu2+ | Fe3+ |
| ��ʼת���������������ʱ��pH | 7.6 | 4.7 | 2.7 |
| ��ȫת���������������ʱ��pH | 9.6 | 6.7 | 3.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�ϣ�����ͭ��CuFeS2��ͨ��8CuFeS2+21O2 8Cu+4FeO+2Fe2O3+16SO2��Ӧ��ȡͭ����������Ļ����
8Cu+4FeO+2Fe2O3+16SO2��Ӧ��ȡͭ����������Ļ����
��1��������Ӧ�У���ԭ��Ϊ ��
��2����ͭ��ұ��ͭ������¯������Fe2O3��FeO��SiO2��Al2O3�����Ʊ�Fe2O3������Ϊ��
����ϡ�����ȡ¯�������ˡ�
����Һ���������ټ������NaOH��Һ�����ˣ�������ϴ�ӡ�������յ�Fe2O3��
��������Ϣ�ش��������⣺
a��ͨ�������ڣ�¯���е�Al2O3����� ��д���ӣ���
b��ѡ���ṩ���Լ������ʵ����֤¯���к���FeO��
�ṩ���Լ���ϡ���� ϡ���� KSCN��Һ ����KMnO4��Һ NaOH��Һ ��ˮ
��ѡ�Լ�Ϊ ��
֤��¯���к���FeO��ʵ������Ϊ ��
��3��������¯���н��к������IJⶨ�������£�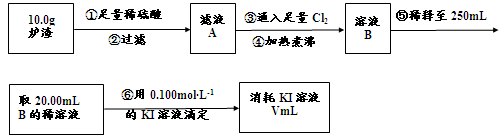
I������۷�����Ӧ�����ӷ���ʽΪ ��
II����������������� ��
III����������õ��IJ����������ձ�������������ͷ�ιܡ� ��
IV�����ζ�����������0.100mol��L?1KI��Һ20.00mL����¯�������İٷֺ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������(��Ҫ�ɷ�ΪAl2O3��������������)����ȡ��������ԭ�ϡ���ȡ�������Ĺ����������£�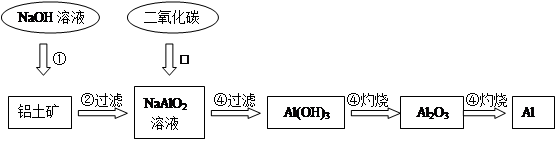
��1���������ӷ���ʽ��ʾ���Ϲ��������еڢٲ���Ӧ��_______ _______��
��2��д�����Ϲ��������еڢ۲���Ӧ�Ļ�ѧ����ʽ��______ ___________��
��3��������������������ڸ����£��ᷢ�����ҵķ�Ӧ���÷�Ӧ�Ļ�ѧ����ʽ_____________�����һ���÷�Ӧ����;________________��
��4�����������������ȡ������������0.9mol���ӷ���ת�ƣ��������ܵõ���������������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ������Fe�ۡ�Cu�ۡ�FeCl3��Һ��CuCl2��Һ�����ij�����г�ַ�Ӧ(�ٶ����������뷴Ӧ)�����ж���������������н���������������ʵĴ��������
(1)��Fe����ʣ�࣬�������в�������____________
(2)��FeCl3��ʣ�࣬�������в�������___________
(3)��CuCl2��ʣ�࣬�������л�������________________________
(4)��FeCl3��CuCl2����ʣ�࣬�������л�������______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������Һ�����������ˣ��õ��������Ƶ���Һ�������Һ��ͨ�������̼�������з�Ӧ�� 2NaAl(OH)4+CO2��2Al(OH)3�� +Na2CO3+H2O
��1���������������зе�������ʵĽṹʽΪ______________�������������е�����Ԫ�ذ�ԭ�Ӹ�����1��1�γɵ����ӻ�����ĵ���ʽΪ__________________��дһ����
��2��AlԪ�صĵ��������ͬ���������������ԣ����о�2����Ҳ�����û�ѧ����ʽ��ʾ��
______________________��__________________________________��
��3������3���ȶ�ͬλ�أ�H뭡� D뮡� T밣��ֱ�Ϊ���a��b��c���������Ԫ�صĽ������ԭ�������ı���ʽΪ______________________________________________��
����ΪH�����������ڱ���A�壬Ҳ�������ڢ�A�壻����ͬѧ��ΪHҲ������̼һ�������ڢ�A�壬��ͬѧ��������__________________________________________________��
��4����֪ͨ�������̼336 L(��״����)������������Al(OH)3 ________________mol,
ʵ��������24 mol Al(OH)3��15 mol Na2CO3��Al(OH)3��������Ҫ�ٵ�ԭ���ǣ�________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������Ƽ���Ͱ�����������̼�Ҫ��ʾ����ͼ��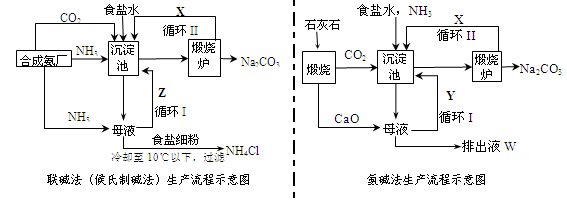
��1�����ַ����ij������о������ķ�Ӧ��ѧ����ʽΪ_____________________________��
��2���������غ�800.00 mol NH3��ˮ��Һ����Ϊ54.00 kg�������Һͨ�������̼����Ӧ��ȫ�����ˣ��õ���Һ31.20kg����NH4HCO3�IJ���Ϊ______________����
��3���ڰ�����������а�Ҫѭ��ʹ�ã�������Ҫ���䣬��ĸҺ�м���ʯ��ǰ��Ҫ���ȵ�ԭ���� ___ ��
��4����������д���Һ����ȡ�Ȼ�茶���Ĺ����Ʋ⣬���ý�����ȷ��_______��ѡ���ţ���
a������ʱ�Ȼ�淋��ܽ�ȱ��Ȼ���С
b��ͨ�백��������NH4+��Ũ�ȣ�ʹ�Ȼ�笠�������
c������ʳ��ϸ�������Na+��Ũ�ȣ� ʹNaHCO3�ᾧ����
d��ͨ�백����ʹNaHCO3ת��ΪNa2CO3�����������NH4Cl����
��5���������ڰ�����Ȼ��������ʴ�70%��ߵ�90%���ϣ���Ҫ�������ѭ�����������һ���ŵ���__________________________________________________��
��6���ӳ����������ľ��庬��NaCl���ʣ�ijͬѧ�ڲⶨ��NaHCO3�ĺ���ʱ����ȡ5.000g���������Ƴ�100mL��Һ���ñ�������Һ�ζ����ü�����ָʾ�������ⶨ���ݼ�¼���£�
| �ζ����� | ����Һ��mL�� | 0.6000mol/L������Һ�������mL�� | |
| ������ | �ն��� | ||
| ��һ�� | 20.00 | 1.00 | 21.00 |
| �ڶ��� | 20.00 | ����ͼ�� | ����ͼ�� |
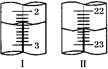 ��ʾ���ĵ�������Һ���Ϊ ��
��ʾ���ĵ�������Һ���Ϊ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��п��ͭ���ɵĺϽ�10 g������������ϡ�����г�ַ�Ӧ��������״��������2��24L������Ͻ���ͭ������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com