
Ā±ĖŲ»Æѧ·įø»¶ą²Ź£¬ÄÜŠĪ³ÉĀ±»ÆĪļ”¢Ā±ĖŲ»„»ÆĪļ”¢¶ąĀ±»ÆĪļµČ¶ąÖÖĄąŠĶµÄ»ÆŗĻĪļ”£
£Ø1£©»łĢ¬äåŌ×ӵļŪµē×ÓÅŲ¼Ź½ĪŖ ”£
£Ø2£©Ā±ĖŲ»„»ÆĪļČēIBr”¢IClµČÓėĀ±ĖŲµ„ÖŹ½į¹¹ĻąĖĘ”¢ŠŌÖŹĻą½ü”£ŌņCl2”¢IBr”¢IClµÄ·ŠµćÓÉøßµ½µĶµÄĖ³ŠņĪŖ ”£
£Ø3£©ĘųĢ¬·ś»ÆĒāÖŠ“ęŌŚ¶ž¾Ū·Ö×Ó(HF)2£¬ÕāŹĒÓÉÓŚ ”£
£Ø4£©I3+ŹōÓŚ¶ąĀ±ĖŲŃōĄė×Ó£¬øł¾ŻVSEPRÄ£ŠĶĶĘ²āI3+µÄæռ乹ŠĶĪŖ £¬ÖŠŠÄŌ×ÓŌÓ»ÆĄąŠĶĪŖ ”£
£Ø5£©¢ŁHClO4”¢¢ŚHIO4”¢¢ŪH5IO6”²æÉŠ“³É(HO)5IO”³µÄĖįŠŌÓÉĒæµ½ČõµÄĖ³ŠņĪŖ £ØĢīŠņŗÅ£©”£
£Ø6£©IBrŗĶĖ®ÄÜ·¢Éś·“Ó¦,Éś³ÉĪļÖŠÓŠŅ»ÖÖĪŖČżŌ×Ó·Ö×Ó,Š“³öøĆ»ÆŗĻĪļµÄ½į¹¹Ź½ ”£
£Ø1£©4s24p5
£Ø2£©IBr£¾ ICl£¾ Cl2
£Ø3£©HF·Ö×Ó¼äŠĪ³ÉĒā¼ü
£Ø4£©VŠĪ£Ø»ņ½ĒŠĪ£©£¬sp3
£Ø5£©¢Ł£¾¢Ś£¾¢Ū
£Ø6£©H-O-I
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©35ŗÅŌŖĖŲäåŌ×Ó»łĢ¬µÄ¼Ūµē×ÓÅŲ¼Ź½ĪŖ4s24p5”££Ø2£©Ā±ĖŲ»„»ÆĪļČēIBr”¢IClµČÓėĀ±ĖŲµ„ÖŹ½į¹¹ĻąĖĘ”¢ŠŌÖŹĻą½ü”£½į¹¹ĻąĖʵÄĪļÖŹ£¬Ļą¶Ō·Ö×ÓÖŹĮæŌ½“󣬷Ö×Ó¼ä×÷ÓĆĮ¦¾ĶŌ½“ó£¬æĖ·ž·Ö×Ó¼ä×÷ÓĆĮ¦Ź¹ĪļÖŹČŚ»Æ»ņĘū»ÆĻūŗĵÄÄÜĮæ¾ĶŌ½øß”£¼ČĪļÖŹµÄČŪµć”¢·Šµć¾ĶŌ½øß”£ÓÉÓŚĻą¶Ō·Ö×ÓÖŹĮæIBr£¾ ICl£¾ Cl2£¬ŌņCl2”¢IBr”¢IClµÄ·ŠµćÓÉøßµ½µĶµÄĖ³ŠņĪŖIBr£¾ ICl£¾ Cl2”££Ø3£©ĘųĢ¬·ś»ÆĒāÖŠ“ęŌŚ¶ž¾Ū·Ö×Ó(HF)2£¬ÕāŹĒÓÉÓŚHF·Ö×Ó¼äŠĪ³ÉĒā¼ü£¬Ōö¼ÓĮĖ·Ö×ÓÖ®¼äµÄĻą»„×÷ÓĆĮ¦”££Ø4£©I3+ŹōÓŚ¶ąĀ±ĖŲŃōĄė×Ó£¬øł¾ŻVSEPRÄ£ŠĶĶĘ²āI3+µÄ½į¹¹ÓėH2OĻąĖĘæռ乹ŠĶĪŖVŠĪ£Ø»ņ½ĒŠĪ£©”£ÖŠŠÄŌ×ÓŌÓ»ÆĄąŠĶĪŖsp3”££Ø5£©HClO4”¢HIO4¶¼ŹĒŌŖĖŲµÄ×īøß¼Ūŗ¬ŃõĖį”£ŌŖĖŲµÄ·Ē½šŹōŠŌŌ½Ē棬Ęä×īøß¼ŪµÄŗ¬ŃõĖįµÄĖįŠŌŌ½Ē攣ÓÉÓŚ·Ē½šŹōŠŌCl£¾I”£ĖłŅŌĖįŠŌ¢ŁHClO4£¾¢ŚHIO4£»¶ų¢ŚHIO4ŗĶ¢ŪH5IO6”²æÉŠ“³É(HO)5IO”³¶¼ŹĒIŌŖĖŲµÄ×īøß¼Ūŗ¬ŃõĖį”£ŌņĖį·Ö×ÓÖŠµÄ·ĒōĒ»łŃõøöŹżŌ½¶ą£¬ĻąÓ¦µÄĖįµÄĖįŠŌ¾ĶŌ½Ē攣ĖłŅŌĖįŠŌ¢ŚHIO4£¾¢ŪH5IO6”£ŌņČżÖÖĖįµÄĖįŠŌÓÉĒæµ½ČõµÄĖ³ŠņĪŖ¢Ł£¾¢Ś£¾¢Ū”££Ø6£©IBrŗĶĖ®·¢Éś·“Ó¦µÄ·½³ĢŹ½ĪŖ£ŗIBr£«H2O=HBr+HIO”£Éś³ÉĪļÖŠĪŖČżŌ×Ó·Ö×ÓHIOµÄ½į¹¹Ź½ĪŖH-O-I”£
æ¼µć£ŗæ¼²éĀ±ĖŲ¼°Ā±»ÆĪļ”¢Ā±ĖŲ»„»ÆĪļ”¢¶ąĀ±»ÆĪļµČµÄÓŠ¹ŲÖŖŹ¶”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
æĘѧ¹¤×÷Õß·¢ĻÖĮķŅ»ÖÖ”°×ćĒņ·Ö×Ó”±N60£¬ĖüµÄ½į¹¹ÓėC60ĻąĖĘ”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
| A£®N60ŗĶC60»„ĪŖĶ¬ĖŲŅģŠĪĢå | B£®N60ŹĒŅ»ÖÖŠĀŠĶ»ÆŗĻĪļ |
| C£®N60ŗĶN2ŹĒĶ¬ĻµĪļ | D£®N60ŗĶ14N¶¼ŹĒµŖµÄĶ¬ĻµĪļ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠŹĀŹµÖŠ£¬Äܹ»Ö¤Ć÷HClŹĒ¹²¼Ū»ÆŗĻĪļµÄŹĒ
| A£®HClŅ×ČÜÓŚĖ® | B£®ŅŗĢ¬µÄHCl²»µ¼µē |
| C£®HCl²»Ņ×·Ö½ā | D£®HClČÜÓŚĖ®ÄܵēĄė£¬³ŹĖįŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø5·Ö£©ÓĆĻą¹ŲµÄ»ÆѧÓĆÓļ±ķŹ¾ĻĀĮŠĪļÖŹ£ŗ
¢Å Š“³öµē×ÓŹ½Al3+ Cl
¢Ę Š“³öĻĀĮŠĪļÖŹµÄ½į¹¹Ź½£ŗN2 CO2
¢Ē ÓƵē×ÓŹ½±ķŹ¾NaOH_______________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(5·Ö£©ŌŚHF”¢H2O”¢NH3”¢CH4”¢CO32-”¢CO2”¢HI·Ö×ÓÖŠ
£Ø1£©CO32-µÄ¼Ū²ćµē×Ó¶ŌµÄæռ乹ŠĶĪŖ
£Ø2£©ŅŌ¼«ŠŌ¼üĻą½įŗĻ£¬¾ßÓŠÕżĖÄĆęĢå½į¹¹µÄ·Ē¼«ŠŌ·Ö×ÓŹĒ ”£
£Ø3£©ŅŌ¼«ŠŌ¼üĻą½įŗĻ£¬¾ßÓŠČż½Ē׶ŠĶ½į¹¹µÄ¼«ŠŌ·Ö×ÓŹĒ ”£
£Ø4£©ŅŌ¼«ŠŌ¼üĻą½įŗĻ£¬¾ßÓŠVŠĶ½į¹¹µÄ¼«ŠŌ·Ö×ÓŹĒ ”£
£Ø5£©ŅŌ¼«ŠŌ¼üĻą½įŗĻ£¬¶ųĒŅ·Ö×Ó¼«ŠŌ×ī“óµÄŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻĀ±ķĪŖÖÜĘŚ±ķµÄŅ»²æ·Ö£¬ĘäÖŠµÄ±ąŗÅ“ś±ķ¶ŌÓ¦µÄŌŖĖŲ”£
ŹŌĢīæÕ”£
(1)Š“³öÉĻ±ķÖŠŌŖĖŲIµÄ»łĢ¬Ō×ӵĵē×ÓÅŲ¼Ź½ŗĶ¼Ū²ćµē×ÓÅŲ¼Ķ¼£ŗ ”£
ŌŖĖŲC”¢D”¢E”¢FµÄµŚŅ»µēĄėÄÜÓɓ󵽊”µÄĖ³ŠņŹĒ (ÓĆŌŖĖŲ·ūŗűķŹ¾)”£
(2)ŌŖĖŲA·Ö±šÓėC”¢D”¢EŠĪ³É×ī¼ņµ„µÄ³£¼ū»ÆŗĻĪļ·Ö×Ó¼×”¢ŅŅŗĶ±ū”£ĻĀĮŠÓŠ¹ŲŠšŹö²»ÕżČ·µÄÓŠ ”£
A£®¼×”¢ŅŅŗĶ±ū·Ö×ÓµÄæռ乹ŠĶ·Ö±šĪŖÕżĖÄĆęĢåŠĪ”¢Čż½Ē׶ŠĪ”¢VŠĪ
B£®¼×”¢ŅŅŗĶ±ū·Ö×ÓÖŠ£¬ÖŠŠÄŌ×Ó¾ł²ÉČ”sp3µÄŌӻƷ½Ź½
C£®ČżÖÖ·Ö×ÓÖŠ¼ü½ĒÓɓ󵽊”µÄĖ³ŠņŹĒ±ū>ŅŅ>¼×
D£®¼×”¢ŅŅŗĶ±ū·Ö×Ó¾łĪŖÓɼ«ŠŌ¼ü¹¹³ÉµÄ¼«ŠŌ·Ö×Ó
(3)ÓÉŌŖĖŲJ”¢C”¢E×é³ÉŅ»ÖÖ»ÆѧŹ½ĪŖJ(CE)5µÄÅäĪ»»ÆŗĻĪļ£¬øĆĪļÖŹ³£ĪĀĻĀ³ŹŅŗĢ¬£¬ČŪµćĪŖ£20.5 ”ę£¬·ŠµćĪŖ103 ”ę£¬Ņ×ČÜÓŚ·Ē¼«ŠŌČܼĮ”£¾Ż“ĖæÉÅŠ¶Ļ£ŗ
¢ŁøĆ»ÆŗĻĪļµÄ¾§ĢåĄąŠĶĪŖ ”£
¢ŚøĆ»ÆŗĻĪļµÄ¾§ĢåÖŠ“ęŌŚµÄ×÷ÓĆĮ¦ÓŠ ”£
A£®Ąė×Ó¼ü
B£®¼«ŠŌ¼ü
C£®·Ē¼«ŠŌ¼ü
D£®·¶µĀ»ŖĮ¦
E£®Ēā¼ü
F£®ÅäĪ»¼ü
¢Ūøł¾Ż¹²¼Ū¼üĄķĀŪŗĶµČµē×ÓĢåĄķĀŪ·ÖĪö£¬CE·Ö×ÓÖŠ¦Ņ¼üÓė¦Š¼üµÄŹżÄæ±ČĪŖ ”£
(4)ŌŚ²ā¶ØAÓėFŠĪ³ÉµÄ»ÆŗĻĪļµÄĻą¶Ō·Ö×ÓÖŹĮæŹ±£¬ŹµŃé²āµĆµÄÖµŅ»°ćøßÓŚĄķĀŪÖµµÄÖ÷ŅŖŌŅņŹĒ ”£
(5)ijŠ©²»Ķ¬×åŌŖĖŲµÄŠŌÖŹŅ²ÓŠŅ»¶ØµÄĻąĖĘŠŌ£¬Čē±ķÖŠŌŖĖŲGÓėŌŖĖŲB£¬ŌŅņŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
A”¢B”¢C”¢DĖÄÖÖŌŖĖŲ“¦ÓŚĶ¬Ņ»¶ĢÖÜĘŚ£¬ŌŚĶ¬×åŌŖĖŲÖŠ£¬AµÄĘųĢ¬Ēā»ÆĪļµÄ·Šµć×īøߣ¬BµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļµÄĖįŠŌŌŚĶ¬ÖÜĘŚÖŠŹĒ×īĒæµÄ£¬CµÄµēøŗŠŌ½éÓŚA”¢BÖ®¼ä£¬DÓėBĻąĮŚ”£
ĒėĢīæÕ£ŗ
£Ø1£©ŌŚBµÄµ„ÖŹ·Ö×ÓÖŠ“ęŌŚ________øö¦Š¼ü£¬________øö¦Ņ¼ü”£
£Ø2£©ŅŃÖŖBµÄĘųĢ¬Ēā»ÆĪļŗÜČŻŅ×ÓėH£«½įŗĻ£¬BŌ×ÓÓėH£«¼äŠĪ³ÉµÄ¼ü½Š________£¬ŠĪ³ÉµÄĄė×ÓĮ¢Ģå¹¹ŠĶĪŖ________£¬ĘäÖŠBŌ×Ó²ÉČ”µÄŌӻƷ½Ź½ŹĒ________”£
£Ø3£©ŌŚA”¢B”¢C”¢DĖÄÖÖŌŖĖŲŠĪ³ÉµÄµē×ÓŹżĻąĶ¬µÄĖÄÖÖĒā»ÆĪļÖŠ£¬·Šµć×īµĶµÄŹĒ________£ØŠ“·Ö×ÓŹ½£©£¬Ęä·ŠµćĻŌÖųµĶÓŚĘäĖūČżÖÖĒā»ÆĪļµÄŌŅņŹĒ£ŗ________________________”£
£Ø4£©AµÄĒā»ÆĪļŅ×ČÜÓŚĖ®£¬¶ųDµÄĒā»ÆĪļÄŃČÜÓŚĖ®£¬ŌŅņŹĒ___________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ā±×åŌŖĖŲµÄµ„ÖŹŗĶ»ÆŗĻĪļŗܶą”£
£Ø1£©Ā±ĖŲŌ×ÓÓėĒāŌ×ÓŠĪ³ÉµÄ¹²¼Ū¼üÖŠ£¬¼«ŠŌ×īĒæ¼üµÄŹĒ_____”£NaF”¢MgF2”¢SiF4ČżÖÖ¾§ĢåµÄČŪµć“Óøßµ½µĶµÄĖ³ŠņŹĒ______”£
£Ø2£©ŅŃÖŖCsICl2²»ĪČ¶Ø£¬ŹÜČČŅ×·Ö½ā£¬ĒćĻņÓŚÉś³É¾§øńÄÜøü“óµÄĪļÖŹ£¬ŌņĖü°“ĻĀĮŠ_____Ź½·¢Éś”£
A£®CsICl2=CsCl+ICl B£®CsICl2=CsI+Cl2
£Ø3£©CH3Cl”¢BF3”¢SCl2ČżÖÖ·Ö×ÓÖŠŹōÓŚ¼«ŠŌ·Ö×ӵďĒ £¬ClO2-ÖŠŠÄĀČŌ×ÓµÄŌӻƹģµĄĄąŠĶĪŖ______£¬Š“³öŅ»ÖÖÓėSO32£»„ĪŖµČµē×ÓĢåµÄ·Ö×Ó ”£
£Ø4£©ŅŃÖŖCaF2¾§Ģå£Ø¼ūĶ¼£©µÄĆܶČĪŖ¦Ńg/cm3£¬NAĪŖ°¢·ü¼ÓµĀĀŽ³£Źż£¬ĻąĮŚ×ī½üµÄ Į½øöCa2+µÄŗĖ¼ä¾ąĪŖa cm£¬ŌņCaF2µÄĻą¶Ō·Ö×ÓÖŹĮææÉŅŌ±ķŹ¾ĪŖ___________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ČēĶ¼ŹĒĀČ»Æļ¤¾§ĢåµÄ¾§°ū£¬ŅŃÖŖ¾§ĢåÖŠ2øö×ī½üµÄCl- Ąė×ÓŗĖ¼ä¾ąĪŖa cm£¬ĀČ»Æļ¤µÄĦ¶ūÖŹĮæĪŖM g£Æmol£¬NAĪŖ°¢·ü¼ÓµĀĀŽ³£Źż£¬ŌņĀČ»Æļ¤¾§ĢåµÄĆܶČĪŖ£Ø £©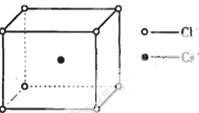
A£® | B£®   | C£® | D£® |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com