
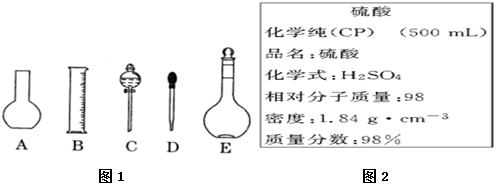
���� ��1�������ù�������һ�����ʵ���Ũ����Һ����ѡ����Ҫ������
��2������ƿ����ϡ��Ũ��Һ�����������ܽ���塢������Һ�ȣ�
��3������m=CVM������Ҫ�����������Ƶ�����������һ�����ʵ���Ũ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ �Ⱦݴ�����
��4������Ũ��Һ����һ�����ʵ���Ũ��ϡ��Һһ�㲽��Ϊ�����㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ �ȣ��ݴ� ѡ����Ҫ������
������C=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ���Ũ�ȣ�������Һϡ���������ʵ����ʵ������������ҪŨ�������������ˮ���ܶȱ�������ܶ�С����������ʱ���������������ˮ������������
�۷������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� ��1���ù�������һ�����ʵ���Ũ����Һһ�㲽��Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���õ��������У�������ƽ��ҩ�ס��ձ���Ͳ����������������ƿ����ͷ�ιܣ�����Ҫ����������ƿ�ͷ�Һ©����Ҫ����450mL��ҺӦѡ��500mL����ƿ��
�ʴ�Ϊ��AC��
��2������ƿֻ����������һ�����ȷŨ�ȵ���Һ���������ƻ��������ƿ������µ����������Һ�壬����ϡ�ͻ��ܽ�ҩƷ���������������ܽ�������ʣ�
��ѡ��BCE��
��3����Ҫ0.1mol/LNaOH��Һ450mL��Ӧѡ��500mL����ƿ����Ҫ������������m=0.1mol/L��40g/mol��0.5L=2.0g��
����һ�����ʵ���Ũ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������ȷ��
����˳��Ϊ��BCAFED��
�ʴ�Ϊ��2.0�� BCAFED��
��4������Ũ��Һ����һ�����ʵ���Ũ��ϡ��Һһ�㲽��Ϊ�����㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ �ȣ��õ�����������Ͳ����ͷ�ιܡ����������ձ���500mL����ƿ�����Ի�ȱ�ٵ�������
500mL������ƿ ��Ͳ��
�ʴ�Ϊ��500mL������ƿ ��Ͳ��
��Ũ�������ʵ���Ũ��C=$\frac{1000��1.84��98%}{98}$=18.4mol/L������ҪŨ�������ΪV����������Һϡ���������ʵ����ʵ�������ã�18.4mol/L��V=0.5mol/L��500mL�����V=13.6mL��
ˮ���ܶȱ�������ܶ�С����������ʱ���������������ˮ�����������Ը�������������ˮ���������Һ��������������49%��
�ʴ�Ϊ��13.6������
��A������ƿ������ˮϴ�Ӻ�δ����������ʵ����ʵ�������Һ������������Ӱ�죬��ҺŨ�Ȳ��䣬��A��ѡ��
B������Ͳ��ȡŨ����ʱ�����ӿ̶��ߣ�����Ũ������Һ���ƫ�����ʵ����ʵ���ƫ����ҺŨ��ƫ�ߣ���Bѡ��
C������Ͳ��ȡŨ�����������ˮ����Ͳϴ�Ӹɾ���ϴ��Һת�Ƶ��ձ��У�������ȡ��Ũ�������ƫ�����ʵ����ʵ���ƫ����ҺŨ��ƫ�ߣ���Cѡ��
D��ת����Һʱ��������������Һ���������²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�����D��ѡ��
E������ʱ����������ƿ�̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���Eѡ��
F�����ݺ�����ƿ����ҡ�ȣ����ź���Һ����ڿ̶��ߣ��ټ�����ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���F��ѡ��
Gδ��ȴ��Һ�����¾Ͷ����ˣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ���Gѡ��
�ʴ�Ϊ��BCEG��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ�������������ǽ���ؼ���ע������ƿ���ѡ��ע���������ķ����ͼ��ɣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
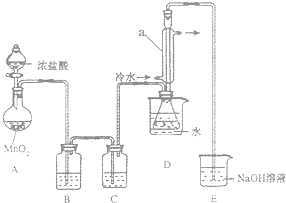 S2Cl2��һ�ֽ��ɫ�ӷ���Һ�壬������������ij��ѧ��ȤС�� �����ʵ���Ʊ�������S2Cl2����������֪S2Cl2��ˮ�������绯��Ӧ��һ������Ԫ�ػ� �ϼ����ߣ���һ���ֻ��ϼ۽��ͣ����������������ʺ��������Cl2��Ӧ��������S2Cl2����Ӧ�Ļ�ѧ����ʽΪ��2S+Cl2$\frac{\underline{\;\;��\;\;}}{\;}$S2Cl2��
S2Cl2��һ�ֽ��ɫ�ӷ���Һ�壬������������ij��ѧ��ȤС�� �����ʵ���Ʊ�������S2Cl2����������֪S2Cl2��ˮ�������绯��Ӧ��һ������Ԫ�ػ� �ϼ����ߣ���һ���ֻ��ϼ۽��ͣ����������������ʺ��������Cl2��Ӧ��������S2Cl2����Ӧ�Ļ�ѧ����ʽΪ��2S+Cl2$\frac{\underline{\;\;��\;\;}}{\;}$S2Cl2��| ���� | S | S2Cl2 |
| �е�/�� | 445 | 138 |
| �۵�/�� | 113 | -76 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �٢ۢ� | C�� | �ۢ� | D�� | �ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 10min�ڣ���Ӧ�ų�������Ϊ197kJ���� | |
| B�� | 10min�ڣ�X��ƽ����Ӧ����Ϊ0.06mol•L-1•min-1 | |
| C�� | ��10minʱ��Y�ķ�Ӧ����С��0.015mol•L-1•min-1��������ϵ�¶Ȳ��䣩 | |
| D�� | ��10minʱ��ZŨ��Ϊ0.6mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �õ��IJ��������У�����������ͷ�ιܡ�500mL����ƿ���ձ� | |
| B�� | ��Ҫ�����������ƹ���1.92g | |
| C�� | û�е���Һ��ȴ��ת�ƽ�����������ҺŨ��ƫ�� | |
| D�� | ϴ��������ƿ����Ҫ�������ʹ�ã���ʹ��ǰ���© |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
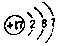 ��
��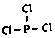 ���������W2���巴Ӧ������1molҺ̬��ʱ�������仯��ͼ2��ʾ��д���÷�Ӧ���Ȼ�ѧ����ʽ$\frac{1}{4}$P4��s��+$\frac{3}{2}$Cl2��g��=PCl3��l����H=-akJ/mol����֪1mol�������W2���巴Ӧ�����ɹ�̬��ʱ������bkJ����1mol��̬��ת��ΪҺ̬��ʱ�ġ�H=��$\frac{1}{4}$b-a��KJ/mol��
���������W2���巴Ӧ������1molҺ̬��ʱ�������仯��ͼ2��ʾ��д���÷�Ӧ���Ȼ�ѧ����ʽ$\frac{1}{4}$P4��s��+$\frac{3}{2}$Cl2��g��=PCl3��l����H=-akJ/mol����֪1mol�������W2���巴Ӧ�����ɹ�̬��ʱ������bkJ����1mol��̬��ת��ΪҺ̬��ʱ�ġ�H=��$\frac{1}{4}$b-a��KJ/mol��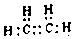 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �þƾ���ȡ��ˮ���嵥�ʵIJ�����ѡ�÷�Һ©���������÷�Һ | |
| B�� | �ܽ����ʱ�ձ���ʹ��ǰ������� | |
| C�� | ��ȡ����Һǰ��Է�Һ©����©����Һ����ʱ����Һ©�����²�Һ�弰�ϲ�Һ������¿ڷų� | |
| D�� | �������ʱ�����������¶ȼƽ��裬�¶ȼ�ˮ����������ƿ��֧�ܿڴ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
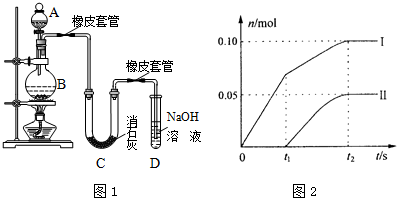
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com