
(1)��֪��
��Fe(s)��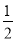 O2(g)=FeO(s)����H����272.0 kJ��mol��1
O2(g)=FeO(s)����H����272.0 kJ��mol��1
��2Al(s)��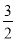 O2(g)=Al2O3(s)����H����1675.7 kJ��mol��1
O2(g)=Al2O3(s)����H����1675.7 kJ��mol��1
Al��FeO�������ȷ�Ӧ���Ȼ�ѧ����ʽ��____________________________________
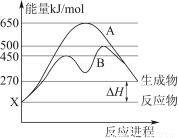
(2)ij���淴Ӧ�ڲ�ͬ�����µķ�Ӧ���̷ֱ�ΪA��B(����ͼ��ʾ)��
������ͼ�жϸ÷�Ӧ�ﵽƽ��������������䣬�����¶ȣ���Ӧ���ת����________(��������������С������������)��
������B���̱����˷�Ӧ���õ�����Ϊ________(ѡ�����)��
A�������¶ȡ������� B������Ӧ���Ũ�� C�������¶� D��ʹ�ô���
(3)1000 ��ʱ���������������������з�Ӧ��Na2SO4(s)��4H2(g) Na2S(s)��4H2O(g)
Na2S(s)��4H2O(g)
�÷�Ӧ��ƽ�ⳣ������ʽΪ________________________________��
��֪K1000 ��<K1200 ������������ϵ�¶ȣ���������ƽ����Է�����������________(��������������С������������)��
(4)�����£����ȡ0.1 mol��L��1 HA��Һ��0.1 mol��L��1 NaOH��Һ��������(��Ϻ���Һ����ı仯���Բ���)����û��Һ��pH��8��
�����Һ����ˮ�������OH��Ũ����0.1 mol��L��1 NaOH��Һ����ˮ�������OH��Ũ��֮��Ϊ________��
����֪NH4A��ҺΪ���ԣ���֪��HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶ�(NH4)2CO3��Һ��pH________7(����<����>����������)����ͬ�¶��£������ʵ���Ũ�ȵ�������������Һ��pH�ɴ�С������˳��Ϊ(�����)________��
a��NH4HCO3 b��NH4A c��(NH4)2CO3 d��NH4Cl
(1)3FeO(s)��2Al(s)=Al2O3(s)��3Fe(s)����H����859.7 kJ/mol
(2)��������D
(3)K��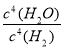 ����С
����С
(4)��107��������c��a��b��d
�������� (1)����ʽ�����١�3�ɵã�3FeO(s)��2Al(s)=Al2O3(s)��3Fe(s)���÷�Ӧ����H����1 675.7 kJ/mol��3��272.0 kJ/mol����859.7 kJ/mol��(2)�������Խ��ͷ�Ӧ�Ļ�ܣ�(3)�����Һ���Ũ�Ȳ�����ƽ�ⳣ������ʽ�У�ƽ�ⳣ��Խ����Ӧ���е�Խ��ȫ���÷�ӦΪ������������䵫�������ӵķ�Ӧ��������ϵ�¶ȣ�ƽ�ⳣ����С��˵��ƽ�����淴Ӧ�����ƶ���������������䵫������С���ʻ�������ƽ����Է����������С��(4)�����Һ��c(H��)��10��8 mol/L��c(OH��)��10��6 mol/L������OH��ȫ����ˮ���룻0.1 mol/L NaOH��Һ��c(OH��)��10��1 mol/L��c(H��)��10��13 mol/L������ˮ���������OH��Ũ�ȵ�����Һ��H��Ũ�ȣ�����Һ����ˮ�������OH��Ũ����0.1 mol��L��1 NaOH��Һ����ˮ�������OH��Ũ��֮��Ϊ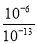 ��107����NH4AΪ���ԣ���֪A����NH4+ˮ��̶���ȣ���HA��Һ�ӵ�Na2CO3��Һ��������ų�����֪H2CO3���Ա�HA������HCO3��ˮ��̶ȱ�A���� ��CO32��ˮ��̶ȱ�HCO3����NH4HCO3��Һ��(NH4)2CO3��Һ���Լ����Ҽ���NH4HCO3��(NH4)2CO3��NH4ClΪǿ�������Σ�������
��107����NH4AΪ���ԣ���֪A����NH4+ˮ��̶���ȣ���HA��Һ�ӵ�Na2CO3��Һ��������ų�����֪H2CO3���Ա�HA������HCO3��ˮ��̶ȱ�A���� ��CO32��ˮ��̶ȱ�HCO3����NH4HCO3��Һ��(NH4)2CO3��Һ���Լ����Ҽ���NH4HCO3��(NH4)2CO3��NH4ClΪǿ�������Σ�������


 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ��ʦ֪ʶ�㾫�� ר��11�绯ѧ��ϰ���������棩 ���ͣ�ѡ����
1 L K2SO4��CuSO4�Ļ����Һ��c(S )=2.0 mol��L-1,��ʯī�缫������Һ,��ͨ��һ��ʱ���,�������ռ���22.4 L(��״��)����,��ԭ��Һ��c(K+)Ϊ�� ��
)=2.0 mol��L-1,��ʯī�缫������Һ,��ͨ��һ��ʱ���,�������ռ���22.4 L(��״��)����,��ԭ��Һ��c(K+)Ϊ�� ��
A.2.0 mol��L-1 B.1.5 mol��L-1
C.1.0 mol��L-1 D.0.5 mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ���ָ�ϰ��ʱ��ѵ ר��8�������Һ��ϰ��B�������棩 ���ͣ������
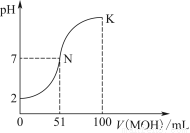
�����£���100 mL 0.01 mol��L��1 HA��Һ����μ���0.02 mol��L��1 MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���(����仯���Բ���)��
�ش��������⣺
(1)��ͼ����Ϣ��֪HAΪ________��(����ǿ����������)��������________________________________________________��
(2)������һ��Ũ�ȵ�MAϡ��Һ��pH��a����a________________________________________________________7
(����>����<����������)�������ӷ���ʽ��ʾ��ԭ��Ϊ_____________________________________________________
��ʱ����Һ����ˮ�������c(OH��)��________��
(3)��д��K������Ӧ����Һ������Ũ�ȵĴ�С��ϵ��_________________________________________��
(4)K���Ӧ����Һ�У�c(M��)��c(MOH)________2c(A��)(����>����<����������)������ʱ��Һ�У�pH��10����c(M��)��c(OH��)��________mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ���ָ�ϰ��ʱ��ѵ ר��8�������Һ��ϰ��A�������棩 ���ͣ�ѡ����
�����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
ʵ���� | c(HA)/mol��L��1 | c(NaOH)/mol��L��1 | ��Ϻ���Һ��pH |
�� | 0.2 | 0.2 | pH��a |
�� | b | 0.2 | pH��7 |
�� | 0.2 | 0.1 | pH<7 |
�� | 0.1 | 0.1 | pH��c |
���жԻ�Ϻ���Һ���й�˵���У�����ȷ����(����)
A�����У���a��7����HA��ǿ��
B��������b��0.2����c(A��)��c(Na��)
C�����У���HA�����ᣬ��c(A��)>c(Na��)>c(H��)>c(OH��)
D��������c��9����c(OH��)��c(HA)��10��9 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ���ָ�ϰ��ʱ��ѵ ר��8�������Һ��ϰ��A�������棩 ���ͣ�ѡ����
25 ��ʱ��Ũ�Ⱦ�Ϊ0.1 mol��L��1��HA��Һ��BOH��Һ��pH�ֱ���1��11������˵����ȷ����(����)
A����0.1 mol��L��1BA��Һ�У�c(A��)��c(H��)��c(BOH)��c(B��)
B������0.1 mol��L��1 BOH��Һϡ����0.001 mol��L��1����Һ��pH��9
C������һ��������������Һ��Ϻ�pH��7������Һ�У�c(A��)>c(B��)
D��������������Һ�������1��1��ϣ�����Һ�У�c(A��)>c(B��)>c(H��)>c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ���ָ�ϰ��ʱ��ѵ ר��7��ѧ��Ӧ���ʺͻ�ѧƽ����ϰ��A�������棩 ���ͣ�ѡ����
���Ĵ�����������Ҫ������������Ӧ��
(��)4NH3(g)��5O2(g) 4NO(g)��6H2O(g)����H����905.5 kJ��mol��1
4NO(g)��6H2O(g)����H����905.5 kJ��mol��1
(��)4NH3(g)��3O2(g) 2N2(g)��6H2O(g)����H����1267 kJ��mol��1
2N2(g)��6H2O(g)����H����1267 kJ��mol��1
����¶ȶ�NO��N2���ʵ�Ӱ����ͼ��ʾ������˵���������(����)
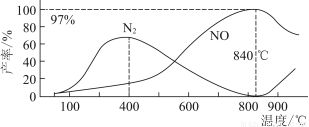
A�������¶ȣ���Ӧ(��)��(��)��ƽ�ⳣ������С
B��840 ���������¶ȣ���Ӧ(��)������Ӧ���ʼ�С����Ӧ(��)������Ӧ��������
C��900 ����NO�����½�����Ҫԭ���Ƿ�Ӧ(��)ƽ�������ƶ�
D��800 ������ʱ�����Ĵ�������Ҫ����(��)����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ���ָ�ϰ��ʱ��ѵ ר��7��ѧ��Ӧ���ʺͻ�ѧƽ����ϰ��A�������棩 ���ͣ�ѡ����
һ�������£���ij�ܱ������м���һ������N2��H2�������淴Ӧ��N2(g)��3H2(g) 2NH3(g)����H����92.2 kJ��mol��1�����0��10 s�ڣ�c(H2)��С��0.75 mol��L��1������˵����ȷ����(����)
2NH3(g)����H����92.2 kJ��mol��1�����0��10 s�ڣ�c(H2)��С��0.75 mol��L��1������˵����ȷ����(����)
A��10 s��15 s��c(NH3)����������0.25 mol��L��1
B��10 s�ڰ�����ƽ����Ӧ����Ϊ0.025 mol��L��1��s��1
C����ƽ����������NH3��v������
D���÷�Ӧ���淴Ӧ�Ļ�ܲ�С��92.2 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ���ָ�ϰ��ʱ��ѵ ר��5��ѧ��Ӧ�е������仯��ϰ���������棩 ���ͣ������
(1)�ڱ���¯�з�����Ӧ��
��Fe2O3(s)��3C(s) 2Fe(s)��3CO(g)����H����492.7 kJ��mol��1
2Fe(s)��3CO(g)����H����492.7 kJ��mol��1
��3CO(g)��Fe2O3(s) 2Fe(s)��3CO2(g)����H����25.2 kJ��mol��1
2Fe(s)��3CO2(g)����H����25.2 kJ��mol��1
��Ӧ2Fe2O3(s)��3C(s) 4Fe(s)��3CO2(g)����H��________kJ��mol��1��
4Fe(s)��3CO2(g)����H��________kJ��mol��1��
(2)��Ȼ��(�Լ����)�ڹ�ҵ��������;�㷺����������ת������H2����Ҫת����Ӧ���£�
CH4 (g)��H2O(g) CO(g)��3H2(g)����H����206.2 kJ��mol��1
CO(g)��3H2(g)����H����206.2 kJ��mol��1
CH4(g)��2H2O(g) CO2(g)��4H2(g)����H����165.0 kJ��mol��1
CO2(g)��4H2(g)����H����165.0 kJ��mol��1
������Ӧ����ԭ�����е�CO��ʹ���ϳɴ����ж��������ȥ����ҵ�ϳ����ô���������CO��ˮ������Ӧ�����׳�ȥ��CO2��ͬʱ�ֿ��Ƶõ�����������ķ������˷�Ӧ��Ϊһ����̼�任��Ӧ���÷�Ӧ���Ȼ�ѧ����ʽ��_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�߿���ѧ���ָ�ϰ��ʱ��ѵ ר��16���ʽṹ��������ϰ���������棩 ���ͣ������
������������ǵĻ������ڿ�ѧ�о���ҵ�����о���������;��
�ش������й����⣺
��1����̬Ni�ĺ�������Ų�ʽΪ__________________________________________��
�ڶ����ڻ�̬ԭ��δ�ɶԵ�������Ni��ͬ�ҵ縺����С��Ԫ����________��
��2�������Ni��CO��n������ԭ�Ӽ۵������������ṩ��������֮��Ϊ18����n��________��CO��N2�ṹ���ƣ�CO��������������������֮��Ϊ________��
��3��NiO��FeO�ľ���ṹ���;����Ȼ��Ƶ���ͬ��
��Ni2����Fe2�������Ӱ뾶�ֱ�Ϊ69 pm��78 pm�����۵�NiO________FeO������<������>������
��NiO������Ni����λ��Ϊ________��
��4������Cu�����백ˮ����������ⶼ���ܷ�Ӧ�������백ˮ���������Ļ����Һ��Ӧ����ԭ����________________________________________________________________________________________________________________________________________________��
��Ӧ�����ӷ���ʽΪ________________________________________________________________________��
��5��һ��ͭ��Ͻ�����������������ܶѻ��Ľṹ���ھ����У�Auԭ��λ�ڶ��㣬Cuԭ��λ�����ģ���úϽ���Auԭ����Cuԭ�Ӹ���֮��Ϊ________�����þ����ı߳�Ϊa pm����Ͻ���ܶ�Ϊ________g��cm��3��ֻҪ������ʽ�����ؼ������ֵ�������ӵ�����ΪNA����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com