
(12��)����ѧһ���ʽṹ�����ʡ�
��֪A��B��C��D��Ϊǰ������Ԫ����ԭ��������������Ԫ��A�Ļ�̬ԭ��2p�����3��δ�ɶԵ��ӣ�Ԫ��B��ԭ�����������������ڲ��������3����Ԫ��C��һ�ֳ�������Ϊ����ɫ��ĩ��D���ڲ���ȫ���������ӣ�������������Ϊl��
��1���ڵ�2�����У���һ�����ܴ���B��Ԫ����____�֡�
��2��A�������̬�⻯����ӵĿռ乹��Ϊ________��H2B���Ҵ��е��ܽ�ȴ���H2C����ԭ����_______��
��3��AB3���У�Aԭ�ӹ�����ӻ�������_______ ����AB3��Ϊ�ȵ��������Ļ�ѧʽ
Ϊ________��д��һ�ּ��ɣ���
��4��D(OH)2������ˮ�������ڰ�ˮ��д�������ڰ�ˮ�����ӷ���
ʽ_______��
( 5)D2B�ľ�����ͼ��ʾ����֪������ܶ�Ϊ 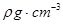 �������ӵ�����Ϊ
�������ӵ�����Ϊ �����߳�Ϊ_______cm(�ú�
�����߳�Ϊ_______cm(�ú�  ��
�� ��ʽ�ӱ�ʾ)��
��ʽ�ӱ�ʾ)��
��1��3����2�������ͣ�ˮ���Ӻ��Ҵ����Ӽ���γ��������3��sp2��CO32����
��4��Cu(OH)2+4NH3?H2O [Cu(NH3)4]2++2OH��+4H2O��( 5)
[Cu(NH3)4]2++2OH��+4H2O��( 5) ��
��
���������������������֪��A��B��C��D��Ϊǰ������Ԫ����ԭ��������������Ԫ��A�Ļ�̬ԭ��2p�����3��δ�ɶԵ��ӣ���AΪ��Ԫ�أ�Ԫ��B��ԭ�����������������ڲ��������3������BΪ��Ԫ�أ�Ԫ��C��һ�ֳ�������Ϊ����ɫ��ĩ����CΪ��Ԫ�أ�D���ڲ���ȫ���������ӣ�������������Ϊl����DΪͭԪ�ء���1��ͬ����Ԫ���������ҵ�һ�����ܳ��������ƣ����ڢ�A��Ԫ��ԭ��p���ΪΪ�����״̬�����ȶ�����һ�����ܱ����ڵĢ�A��͢�A��Ԫ�ش��ڵ�2�����У���һ�����ܴ�������Ԫ����N��F��Ne��3�֡���2�����������̬�⻯�ﰱ���ӵĿռ乹��Ϊ�����ͣ�H2O���Ҵ��е��ܽ�ȴ���H2S����ԭ����ˮ���Ӻ��Ҵ����Ӽ���γ��������3��NO3���У�Nԭ����3�Ե��ӣ����Ӵ�1������ɣ���������ӻ�������sp2����NO3����Ϊ�ȵ��������Ļ�ѧʽΪCO32���� ��4��Cu(OH)2������ˮ�������ڰ�ˮ��������Ϸ�Ӧ�����ӷ���ʽΪCu(OH)2+4NH3?H2O [Cu(NH3)4]2++2OH��+4H2O��( 5)��Cu2O�ľ��������и������1����������Cu2����4����O2����8��1/8+1=2�����þ�������=2��160/NAg���þ������ܶ�Ϊ�� g?cm-3�����߳�a=
[Cu(NH3)4]2++2OH��+4H2O��( 5)��Cu2O�ľ��������и������1����������Cu2����4����O2����8��1/8+1=2�����þ�������=2��160/NAg���þ������ܶ�Ϊ�� g?cm-3�����߳�a=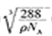 cm��
cm��
���㣺�������ʽṹ�����ʣ��漰ԭ�ӽṹ��ԭ�Ӽ�ijɼ���ʽ������Ԫ�������ɼ�����ṹ���������㡣


 ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д� �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣�.
��. �±��г���A~R 9��Ԫ�������ڱ��е�λ��(��Ԫ�ط���)��
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | | | | E | | F | | |
| 3 | A | C | D | | | | G | R |
| 4 | B | | | | | | H | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
[���ʽṹ�����ʣ�13��]
��1����ͭͬ���ڡ���̬ԭ��������������ͬ�Ĺ���Ԫ�أ����̬ԭ�ӵĵ����Ų�ʽ ��
��2����ͼ���߱�ʾ���ֶ�����Ԫ�ص�ԭ��������������˳�����У����䳣�����ʷе�Ĺ�ϵ������A���ʾ�ĵ����� ���ѧʽ����
| ����/(pm) | B��F | B��Cl | B��Br |
| ����ֵ | 152 | 187 | 199 |
| ʵ��ֵ | 130 | 175 | 187 |
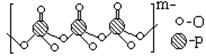
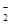 ���ж�HF
���ж�HF ��HF
��HF �����ܷ��γ��������˵�����ɡ�
�����ܷ��γ��������˵�����ɡ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣�����A��B��C��D��E��F��G��H���ֶ���������Ԫ�أ�ԭ����������������֪A��E��D��G�ֱ�ͬ���壻E��F��G��Hͬ���ڣ�A�ֱ���C��D���γɺ���10�����ӵĹ��ۻ�����M��N��B������������������Ӳ�����2����D�ǵؿ��к�������Ԫ�أ�Fλ��B��ǰһ���塣��ش��������⣺
��1��Ԫ��B�����ڱ��е�λ�� ��M�Ŀռ乹���� ��
��2��A��D��E����Ԫ�����һ�ֳ��������W��û�����������Ӿ�����ͬ��ԭ���������Ŀ�Ҳ����磬W�ĵ���ʽΪ ����ҵ������ijһ����Ӧ��ͬʱ�����û������H�ĵ��ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3��E��FԪ�ص�����������Ӧ��ˮ����֮�䷴Ӧ�����ӷ���ʽ ��
��4��M��N���ܽ��H+�����н��H+������ǿ���� ���ѧʽ���������ӷ���ʽ֤�� ��
��5��E�ֱ���D��G�γ�Ħ��������ȵĻ�����X��Y������Y��ˮ��Һ�Լ��Ե�ԭ���� �������ӷ���ʽ��ʾ����������7.8 g X��ˮ��Ӧ�ų�Q kJ������Q��0����д���÷�Ӧ���Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣�����ѧ��ѡ��3�����ʽṹ�����ʡ�
��a��b��c��d��f����ǰ������Ԫ�أ�ԭ��������������a��b��c����Ԫ�صĻ�̬ԭ�Ӿ�����ͬ���ܲ���ܼ�����һ������I1(a)<I1(c)<I1(b)�������л�̬bԭ�ӵ�2p������ڰ����״̬�� dΪ���ڱ�ǰ�������е縺����С��Ԫ�أ�f��ԭ������Ϊ29����ش��������⡣(�����ʾ����Ԫ��������Ӧ��Ԫ�ط���)
��1��д��ac2�ĵ���ʽ__________����̬fԭ�ӵ���Χ�����Ų�ʽΪ ��
��2��д��һ����ac2��Ϊ�ȵ���������ʵĻ�ѧʽ ��
��3��b�ļ��⻯��ķе��ͬ��Ԫ���⻯��ķе� ������ߡ��͡���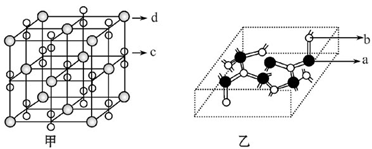
��4��������M��c��d����Ԫ����ɣ��侧���ṹ��ף���M�Ļ�ѧʽΪ ��
��5��������N�IJ��ֽṹ���ң�N��a��b��Ԫ����ɣ���Ӳ�ȳ������ʯ���Իش𣺢�N�ľ�������Ϊ________________________����Ӳ�ȳ������ʯ��ԭ����___________________��
��N������a��b��Ԫ��ԭ�ӵ��ӻ���ʽ��Ϊ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣�Q��W��X��Y��Z��ԭ��������������Ķ�����Ԫ�أ�X��Y�ǽ���Ԫ�أ�Q��W��Z�Ƿǽ���Ԫ�ء�����Ԫ�غ˵����֮��Ϊ55����Ӧԭ������������֮��Ϊ21��W��Z������������ͬ����Z�ĺ˵������W��2����
��1��Q�����ڱ���λ�ڵ� ���� �塣
��2��X��Y���Ե�����������Ӧ��ˮ������Է�����Ӧ�����κ�ˮ����д���÷�Ӧ�����ӷ���ʽ�� ��
��3��X��������W������ȼ�տ����ɻ�����R��R�ĵ���ʽ ___�������������еĻ�ѧ��������Ϊ ��
��4��Z���⻯����W���⻯�����Ӧ����Z���ʺ�ˮ��д���仯ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣���֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵�����������ӡ������Ϣ���±���ʾ�������ƶϻش��������⣺������ʱA��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
| A | A������������Ӧ��ˮ���ﻯѧʽΪH2AO3 |
| B | BԪ�صĵ�һ�����ܱ�ͬ������������Ԫ�ض��� |
| C | Cԭ����ͬ����ԭ���а뾶���ϡ��������⣩���䵥����ɫΪ��ɫ |
| D | Z�Ļ�̬ԭ�����������Ų�ʽΪ3s23p2 |
| E | E��Cλ�ڲ�ͬ���ڣ�Eԭ�Ӻ���������������C��ͬ�����������Ӿ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺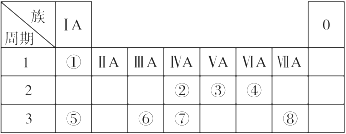
��1��������Ԫ�ص�ԭ���У�ԭ�Ӱ뾶������ ����Ԫ�ط��ţ���
��2���ؿ��к������ڵڶ�λ��Ԫ�������ڱ��е�λ���� ��
��3���١��ܡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ����д�����ֻ�����ĵ���ʽ �� ��
��4��W�ǵ����������ͬ�����Ԫ�ء��ݴ��Ʋ�W�����ܾ��е������� ��
| A����������ϼ�Ϊ��6�� | B����̬�⻯���H2S�ȶ� |
| C������������ˮ��������Ա������� | D�������ڳ����¿����������� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ʰ���Ҫ���ɸߵ��͵�˳������
��1��NaF��NaCl�������ᡢSiC�������ʵ��۷е�˳�� ��
��2��C��N��O����Ԫ�صĵ�һ�����ܣ� ��
��3��H2O����Է���������H2SС�����е�ȴ�ߵ�ԭ�� ��
��4��ij��̬�Ŵط��ӵķ��ӽṹ��ͼ��ʾ���侧��ľ�����CO2����ľ���������ͬ������Ŵط��ӵķ���ʽΪ ��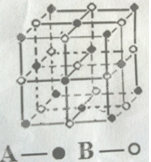
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com