
�����������ʵ���Ҫ���������ʽṹ����ش��������⡣
��1����֪A��BΪ��������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ��������±���ʾ:
������/kJ��mol-1 | I1 | I2 | I3 | I4 |
A | 578 | 1817 | 2745 | 11578 |
B | 738 | 1451 | 7733 | 10540 |
Aͨ���� �ۣ�BԪ�صĺ�������Ų�ʽΪ ��
��2��ʵ��֤��:KCl��MgO��CaO��TiN��4�־���Ľṹ��NaCl����ṹ����(����ͼ��ʾ)����֪3�����Ӿ���ľ������������±�:
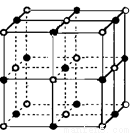
���Ӿ��� | NaCl | KCl | CaO |
������/kJ��mol-1 | 786 | 715 | 3401 |
���4�����Ӿ���(������NaCl)�۵�Ӹߵ��͵�˳���� ������MgO������һ��Mg2+��Χ�������ڽ��ҵȾ����Mg2+�� ����
��3�����������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á�������������V2O5��CrO2�У��ʺ���¼�����ŷ�ԭ�ϵ��� ��
��4��ij�����ķ��ӽṹ����ͼ��ʾ��������ڲ����� (�����)��
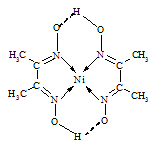
A�����Ӽ�
B�����Լ�
C��������
D�����
E.���
F.�Ǽ��Լ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ʡ��һ��ѧ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
������������һ��ȡ����ֻ���������ַе㲻ͬ�IJ�����ǣ� ��
 A��CH3CH2CH3
A��CH3CH2CH3
B��CH3CH3
C��CH3C H2CH2 CH2CH3
D��CH3C H2CH2CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�긣��ʡ��һ��ѧ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
���й����л����������ʶ��ȷ���ǣ� ��
A��ʯ�͵ķ���ú�ĸ��������������仯
B�����ۡ���ά�ء���֬�������ʶ�����Ȼ�л��߷��ӻ�����
C����������Һ���������Һ������ͭ��Һ�����ij�������ԭ����ͬ
D��ֲ������ʹ������Ȼ�̼��Һ��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ�߶���ѧ�ڵ��Ĵ��¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
������ʵ���Ũ�ȵ������NH4Cl�����Һ����μ���NaOH��Һ(����)����Ӧ�����У��������ӷ���ʽ����ʵ��������ǣ� ��
A.H++OH-=H2O
B.H++NH4++2OH-=NH3��+2H2O
C.4H++NH4++5OH-=NH3��+5H2O
D.H++2NH4++3OH- =2NH3��+3H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ�߶���ѧ�ڵ��Ĵ��¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��NA��ʾ����٤��������ֵ������������ȷ���ǣ� ��
A�����³�ѹ�µ�33.6L������27g����ַ�Ӧ��ת�Ƶ�����Ϊ3NA
B��7.8gNa2O2��������������������Ϊ0.4NA
C����CO2��O2��ɵĻ�����й���NA�����ӣ����е���ԭ������Ϊ2NA
D��1LŨ��Ϊ1mol/L��Na2CO3��Һ�к���NA��CO32-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ�����и߶���ѧ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
���и���������ָ����Һ���ܴ����������( )
��pH��11����Һ�У�CO32-��Na+��AlO2-��NO3-
����ɫ��Һ�У�K+��Na+��MnO4-��SO42-
�ۼ���Al�ܷų�H2����Һ�У�Cl����HCO3-��SO42-��NH4+
��������Һ�У�Fe2+��Al3+��NO3-��Cl��
����ˮ�������c(OH��)��1��10��13 mol��L��1����Һ�У�Na+��Ba2+��Cl����Br��
A���٢� B���ڢ� C���ڢ� D���ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ�����и߶���ѧ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
���л������к�������̼ԭ�ӵ���( )
A.  B.
B. 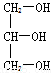 C. CH3CH2OH D.
C. CH3CH2OH D. 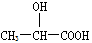
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�긣��ʡ�����и�һ��ѧ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
��1mol N2O5����2L�ܱ������У���һ���¶��·������з�Ӧ��
��2N2O5(g)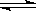 2N2O4 (g)��O2 (g)�� ��N2O4(g)
2N2O4 (g)��O2 (g)�� ��N2O4(g)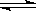 2NO2 (g) �ﵽƽ��ʱ��
2NO2 (g) �ﵽƽ��ʱ��
c(O2)��0.2 mol��L��1��c(NO2)��0.6mol��L��1������¶��·�Ӧ�ٵ�ƽ�ⳣ��Ϊ( )
A��3.2 B��0.2 C��1/180 D��4/45
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�긣��ʡ�����и߶���ѧ����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
ij�ס���������̬���������м���Է�������С���ң����������ܶ�����ͬ״����H2�ܶȵ�13�����ѱ�״����4.48L�û������ͨ����������ˮ�У���ˮ����2.8g���������й�����������ǣ� ��
A����һ��������
B���ҵ����ʵ���Ϊ0.05mol
C�������������ҵĿ��ܽṹ��3�֣�����˳���칹��
D��������Ϊ2.4g
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com