
(10��)��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ���£�(��ʾ��A�����пɱ��̬������Ӧ���ɵͼ�̬)
��D��Һ�����ˮ�пɵõ���FΪ��ɢ�ʵĺ��ɫ���塣��ش��������⣺
(1)���ɫ����F����ֱ����С�ķ�Χ��________��
(2)B�Ļ�ѧʽ��________��
(3)д����D����Һ�백ˮ��Ӧ�����ӷ���ʽ��
________________________________________________________________________��
C����Һ��˫��ˮ��Ӧ�����ӷ���ʽ��
________________________________________________________________________��
(4)д������E�������ӵ�ʵ�鷽��������
________________________________________________________________________��
 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
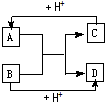 ��֪A��B��C��D�ֱ�����ѧ��ѧ�г��������ֲ�ͬ���ӣ�����֮��������ͼ��ʾ��Ӧ��ϵ��
��֪A��B��C��D�ֱ�����ѧ��ѧ�г��������ֲ�ͬ���ӣ�����֮��������ͼ��ʾ��Ӧ��ϵ�� ��
��
 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��³����1�ۺϾ� ���ͣ�ʵ����
(10��)��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ���£�(��ʾ��A�����пɱ��̬������Ӧ���ɵͼ�̬)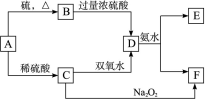
��D��Һ�����ˮ�пɵõ���FΪ��ɢ�ʵĺ��ɫ���塣��ش��������⣺
(1)���ɫ����F����ֱ����С�ķ�Χ��________��
(2)B�Ļ�ѧʽ��________��
(3)д����D����Һ�백ˮ��Ӧ�����ӷ���ʽ��
________________________________________________________________________��
C����Һ��˫��ˮ��Ӧ�����ӷ���ʽ��
________________________________________________________________________��
(4)д������E�������ӵ�ʵ�鷽��������
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��³����1�ۺϾ� ���ͣ�����
(10��)��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ���£�(��ʾ��A�����пɱ��̬������Ӧ���ɵͼ�̬)
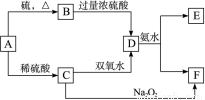
��D��Һ�����ˮ�пɵõ���FΪ��ɢ�ʵĺ��ɫ���塣��ش��������⣺
(1)���ɫ����F����ֱ����С�ķ�Χ��________��
(2)B�Ļ�ѧʽ��________��
(3)д����D����Һ�백ˮ��Ӧ�����ӷ���ʽ��
________________________________________________________________________��
C����Һ��˫��ˮ��Ӧ�����ӷ���ʽ��
________________________________________________________________________��
(4)д������E�������ӵ�ʵ�鷽��������
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com