
ŌŚĻĀĮŠø÷±ä»ÆÖŠ£¬EĪŖĪŽÉ«ĪŽĪ¶µÄŅŗĢå£Ø³£ĪĀĻĀ£©£¬FĪŖµ»ĘÉ«·ŪÄ©£¬GĪŖ³£¼ūµÄĪŽÉ«ĘųĢå£Ø·“Ó¦Ģõ¼ž¾łŅŃŹ”ĀŌ£©”£»Ų“šĻĀĮŠĪŹĢā£ŗ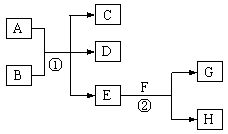
£Ø1£©ŌŚ·“Ó¦¢ŚÖŠ£¬ĆæÉś³É 2£®24L ĘųĢåG£Ø±ź×¼×“æö£©Ź±£¬øĆ·“Ó¦×ŖŅʵē×ÓµÄĪļÖŹµÄĮæŹĒ mol”£
£Ø2£©Čō·“Ó¦¢ŁŌŚ¼ÓČČĢõ¼žĻĀ½ųŠŠ£¬µ„ÖŹAŗĶ»ÆŗĻĪļB°“ĪļÖŹµÄĮæÖ®±ČĪŖ1:2·¢Éś·“Ó¦£¬ĒŅC”¢DŹĒĮ½ÖÖ¾łÄÜŹ¹³ĪĒåµÄŹÆ»ŅĖ®±ä»ė×ĒµÄĪŽÉ«ĘųĢ壬Ōņ·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø3£©Čō·“Ó¦¢ŁŌŚČÜŅŗÖŠ½ųŠŠ£¬AŹĒŅ»ŌŖĒæ¼ī£¬BŹĒŅ»ÖÖĖįŹ½ŃĪ£¬DŹĒŅ» ÖÖŹ¹ŹŖČóŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬ĒŅBÓöŃĪĖįÄÜÉś³ÉŹ¹Ę·ŗģČÜŅŗĶŹÉ«µÄĘųĢ唣ŌŚ¼ÓČČĢõ¼žĻĀ£¬µ±A¹żĮæŹ±£¬·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½ŹĒ ”£
( 4)ÓɽšŗģŹÆ(TiO2)ÖĘČ”µ„ÖŹTi£¬Éę¼°µ½µÄ²½ÖčĪŖ£ŗTiO2”śTiCl4 Ti
Ti
¢ŁC(s) + O2(g) = CO2(g)£» ”÷H = £393£®5 kJ?mol-1
¢Ś2CO(g) + O2(g) = 2CO2(g)£» ”÷H = £566 kJ?mol-1
¢ŪTiO2(s) + 2Cl2(g) = TiCl4(s) + O2(g)£» ”÷H =" +141" kJ?mol-1
ŌņTiO2(s) + 2Cl2(g) + 2C(s)= TiCl4(s) + 2CO(g) µÄ”÷H = _________________”£
£Ø1£©0£®2 £Ø2£©C+2H2SO4£ØÅØ£© CO2”ü+2SO2”ü+2H2O£®
CO2”ü+2SO2”ü+2H2O£®
(3) NH4++HSO3-+2OH- NH3”ü+2H2O+SO32-£® (4)”÷H=”Ŗ80KJ/mol£®
NH3”ü+2H2O+SO32-£® (4)”÷H=”Ŗ80KJ/mol£®
½āĪöŹŌĢā·ÖĪö£ŗ(1)øł¾ŻĢāŅāæÉÖŖ£ŗE”¢F”¢G”¢H·Ö±šŹĒE£ŗH2O£»F£ŗNa2O2;G: O2;H: NaOH£® ·“Ó¦¢ŚµÄ·½³ĢŹ½ŹĒ2H2O+2 Na2O2= O2”ü+NaOH£® æɼūĆæ²śÉś1molµÄŃõĘų£¬×ŖŅʵē×Ó2mol£®ĻÖŌŚÉś³ÉO2Ģå»żŹĒ2£®24L £¬Ęę0£®1mol,ĖłŅŌ×ŖŅʵē×Ó0£®2mol£®(2)øł¾ŻĢāŅāæÉÖŖ£ŗA£ŗC£»B£ŗÅØH2SO4£»C£ŗCO2£»D£ŗSO2”£·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½ŹĒC+2H2SO4£ØÅØ£© CO2”ü+2SO2”ü+2H2O£®£Ø3£©øł¾ŻĢāŅāæÉÖŖ£ŗA£ŗNaOH£»B£ŗNH4HSO3£»C£ŗH2O£»D£ŗNH3”£E£ŗNa2SO3£® ŌŚ¼ÓČČĢõ¼žĻĀ£¬µ±A¹żĮæŹ±£¬·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½NH4++HSO3-+2OH-
CO2”ü+2SO2”ü+2H2O£®£Ø3£©øł¾ŻĢāŅāæÉÖŖ£ŗA£ŗNaOH£»B£ŗNH4HSO3£»C£ŗH2O£»D£ŗNH3”£E£ŗNa2SO3£® ŌŚ¼ÓČČĢõ¼žĻĀ£¬µ±A¹żĮæŹ±£¬·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½NH4++HSO3-+2OH- NH3”ü+2H2O+SO32-£®¢Ł”Į2-¢Ś+¢ŪæÉµĆ£ŗTiO2(s) + 2Cl2(g) + 2C(s)= TiCl4(s) + 2CO(g)”÷H =”Ŗ80KJ/mol£®
NH3”ü+2H2O+SO32-£®¢Ł”Į2-¢Ś+¢ŪæÉµĆ£ŗTiO2(s) + 2Cl2(g) + 2C(s)= TiCl4(s) + 2CO(g)”÷H =”Ŗ80KJ/mol£®
æ¼µć£ŗæ¼²éŌŖĖŲµÄĶʶĻ¼°ŌŖĖŲÓė»ÆŗĻĪļµÄŠŌÖŹµÄÖŖŹ¶”£


 »Ŗ¶«Ź¦“ó°ęŅ»æĪŅ»Į·ĻµĮŠ“š°ø
»Ŗ¶«Ź¦“ó°ęŅ»æĪŅ»Į·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌŚĻĀĶ¼ĖłŹ¾µÄĪļÖŹ×Ŗ»Æ¹ŲĻµÖŠ£¬AŹĒŗ£Ė®ÖŠŗ¬Įæ×ī·įø»µÄŃĪ£¬BŹĒ³£¼ūµÄĪŽÉ«ŅŗĢ壬FŌŚDÖŠČ¼ÉÕ·¢³ö²Ō°×É«»šŃę”£HæÉÓĆÓŚÖĘŌģ¹āµ¼ĻĖĪ¬£¬JŹĒŅ»ÖÖČé°×É«Äż½ŗד³Įµķ”££Ø²æ·ÖÉś³ÉĪļŗĶ²æ·Ö·“Ó¦Ģõ¼žĪ“ĮŠ³ö£©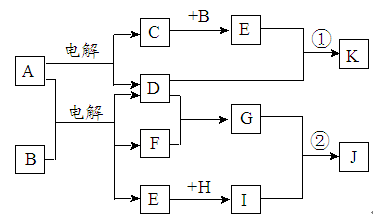
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©AµÄµē×ÓŹ½ĪŖ ”£
£Ø2£©HµÄ»ÆѧŹ½ĪŖ ”£
£Ø3£©Š“³ö·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½ ”£
£Ø4£©Š“³ö·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻĀĶ¼ŹĒÓɶĢÖÜĘŚŌŖĖŲ×é³ÉµÄŅ»Š©µ„ÖŹ¼°Ęä»ÆŗĻĪļÖ®¼äµÄ×Ŗ»Æ¹ŲĻµĶ¼”£ø÷·½æņ±ķŹ¾ÓŠ¹ŲµÄŅ»ÖÖ·“Ó¦Īļ»ņÉś³ÉĪļ£ØijŠ©ĪļÖŹŅŃ¾ĀŌČ„£©£¬ĘäÖŠA”¢B”¢DŌŚ³£ĪĀĻĀ¾łĪŖĪŽÉ«ĪŽ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢ壬CŹĒŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬MŹĒ×ī³£¼ūµÄĪŽÉ«ŅŗĢ唣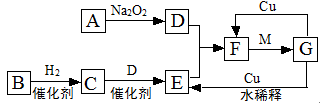
£Ø1£©ĪļÖŹGµÄ»ÆѧŹ½£ŗ ”£
£Ø2£©ĪļÖŹBµÄµē×ÓŹ½£ŗ ”£
£Ø3£©Š“³öC”śEµÄ»Æѧ·½³ĢŹ½£ŗ £»
£Ø4£©G”śFµÄĄė×Ó·½³ĢŹ½£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
A”¢B”¢C”¢D”¢E”¢FĪŖ֊ѧ»Æѧ֊µÄ³£¼ūĪļÖŹ£¬ĒŅĪļÖŹAÓÉ1~2ÖÖ¶ĢÖÜĘŚŌŖĖŲ×é³É£¬ŌŚŅ»¶ØĢõ¼žĻĀÓŠČēĻĀ×Ŗ»Æ¹ŲĻµ£¬ĒėĶź³ÉĻĀĮŠĪŹĢā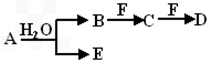
£Ø1£©Čō³£ĪĀĻĀAĪŖÓŠÉ«ĘųĢå
¢ŁČōFŹĒŅ»ÖÖ½šŹōµ„ÖŹ£¬ĒėŠ“³öBŗĶŹŹĮæF·“Ӧɜ³ÉCÓėĘųĢåEµÄĄė×Ó·½³ĢŹ½£ŗ_________________ _____________”£
¢ŚČōCĪŖÖ±ĻߊĶ·Ö×Ó£¬E¾ßÓŠĘư׊Ō£¬ĪļÖŹFŃęÉ«·“Ó¦³Ź»ĘÉ«£¬ĒėŠ“³ö¹¤ŅµÉĻÖʱøĪļÖŹFµÄ»Æѧ·“Ó¦·½³ĢŹ½£ŗ_______ ____________£»__________ _____”£
£Ø2£©ČōAĪŖµ»ĘÉ«¹ĢĢ壬ŌņAĪļÖŹµÄµē×ÓŹ½ĪŖ£ŗ_________________”£
¢ŁČōĪļÖŹBŗĶDµÄĻą¶Ō·Ö×ÓÖŹĮæĻąµČ£¬ĪļÖŹCµÄ“óĮæÅÅ·ÅĶłĶł»įŌģ³ÉŃĻÖŲµÄ»·¾³ĪŹĢā¼“_____ _____£»
¢ŚČōĪļÖŹAŗĶDµÄĻą¶Ō·Ö×ÓÖŹĮæĻąµČ,ĒėÓĆĄė×Ó·½³ĢŹ½±ķŹ¾ĪļÖŹFµÄĖ®ČÜŅŗ³ŹĖįŠŌµÄŌŅņ_____ _____”£
£Ø3)ČōĪļÖŹAÖŠŅ»ÖÖŌŖĖŲŌ×ÓµÄ×īĶā²ćµē×ÓŹżĪŖÄŚ²ćµē×Ó×ÜŹżµÄ1/5£¬½«BŗĶD·Ö±šČÜÓŚĖ®£¬ĖłµĆČÜŅŗ°“Ē”µ±±ČĄż»ģŗĻ£¬æɵĆŅ»²»ŗ¬½šŹōŌŖĖŲµÄŃĪČÜŅŗ£¬ĒėŠ“³öAµÄ»ÆѧŹ½________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
·Ē½šŹōµ„ÖŹA£¬¾ČēĻĀĶ¼ĖłŹ¾µÄ¹ż³Ģ×Ŗ»ÆĪŖŗ¬ŃõĖįD£¬ŅŃÖŖDĪŖ³£¼ūĒæĖį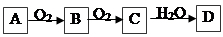
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ČōAŌŚ³£ĪĀĻĀĪŖ¹ĢĢ壬BŹĒÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«µÄÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĪŽÉ«ĘųĢå
¢ŁDµÄ»ÆѧŹ½ŹĒ
¢ŚŌŚ¹¤ŅµÉś²śÖŠBĘųĢåµÄ“óĮæÅŷű»ÓźĖ®ĪüŹÕŗóŠĪ³ÉĮĖ ¶ųĪŪČ¾ĮĖ»·¾³
£Ø2£©ČōAŌŚ³£ĪĀĻĀĪŖĘųĢ壬CŹĒŗģ×ŲÉ«ĘųĢ唣¢ŁAŗĶCµÄ»ÆѧŹ½·Ö±šŹĒ£ŗA £»C
¢ŚDµÄÅØČÜŅŗŌŚ³£ĪĀĻĀæÉÓėĶ·“Ó¦²¢Éś³ÉCĘųĢ壬ĒėŠ“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻĀĶ¼ø÷ĪļÖŹŹĒ֊ѧ»Æѧ֊³£¼ūµÄĪļÖŹ£¬¼×”¢ŅŅ¾łŹĒĄė×Ó»ÆŗĻĪļ£¬ĒŅŅõ”¢ŃōĄė×ÓøöŹż±ČĪŖ1”Ć1”£¼×æÉ×÷·¢½Ķ·Ū£¬ŅŅŹĒŅ»ÖÖ³£ÓĆµÄ»Æ·Ź”£B”¢D³£ĪĀĻĀŹĒĘųĢ唣Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¼×µÄĖ×ĆūŹĒ____________”£
£Ø2£©DµÄĖ®ČÜŅŗµĪČė·ÓĢŖŹŌŅŗŗó£¬ČÜŅŗĻŌŗģÉ«£¬ĒėÓƱŲŅŖµÄĪÄ×Ö¼ÓŅŌ½āŹĶ²¢Š“³öĻą¹ŲµÄĄė×Ó·½³ĢŹ½£ŗ___ _________”£
£Ø3£©¼×ČÜŅŗÖŠ¼ÓČėĀČ»ÆĀĮČÜŅŗ£¬æÉŅŌ¹Ū²ģµ½µÄĻÖĻóĪŖ ŹŌŠ“³ö·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ____
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻĀĶ¼ŹĒĪŽ»śĪļA”«MŌŚŅ»¶ØĢõ¼žĻĀµÄ×Ŗ»Æ¹ŲĻµ£Ø²æ·Ö²śĪļ¼°·“Ó¦Ģõ¼žĪ“ĮŠ³ö£©”£ĘäÖŠ£¬IŹĒµŲæĒÖŠŗ¬Įæ×īøߵĽšŹō£¬KŹĒŅ»ÖÖŗģ×ŲÉ«ĘųĢ唣 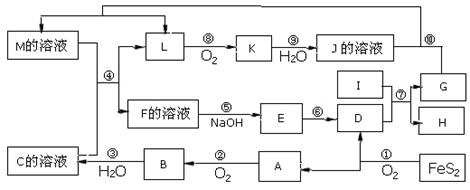
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©×é³Éµ„ÖŹIµÄŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆĪŖ ”£
£Ø2£©Óū¼ģŃéFČÜŅŗÖŠŹĒ·ńŗ¬ÓŠÉŁĮæM£¬æÉŃ”ŌńµÄŹŌ¼ĮĪŖ £ØĢī»ÆѧŹ½£©”£
£Ø3£©ŌŚ·“Ó¦¢ßÖŠ»¹Ō¼ĮÓėŃõ»Æ¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ___________________”£
£Ø4£©Ä³Ķ¬Ń§Č”FµÄČÜŅŗ£¬Ėį»Æŗó¼ÓČėKI”¢µķ·ŪČÜŅŗ£¬±äĪŖĄ¶É«”£Š“³öÓėÉĻŹö±ä»Æ¹ż³ĢĻą¹ŲµÄĄė×Ó·½³ĢŹ½£ŗ ”£
£Ø5£©½«»ÆŗĻĪļD ÓėKNO3”¢KOH ¹²ČŚ£¬æÉÖʵĆŅ»ÖÖ”°ĀĢÉ«”±»·±£øߊ§¾»Ė®¼ĮK2FeO4£ØøßĢśĖį¼Ų£©.Ķ¬Ź±»¹Éś³ÉKNO2ŗĶH2O ”£øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ__________________________”£
£Ø6£©Ć¾ÓėIµÄŗĻ½šŹĒŅ»ÖÖĒ±ŌŚµÄÖüĒā²ÄĮĻ£¬æÉŌŚė²Ęų±£»¤ĻĀ£¬½«Ņ»¶Ø»Æѧ¼ĘĮæ±ČµÄMg”¢Iµ„ÖŹŌŚŅ»¶ØĪĀ¶ČĻĀČŪĮ¶»ńµĆ”£
¢ŁČŪĮ¶ÖʱøĆ¾IŗĻ½šŹ±ĶØČėė²ĘųµÄÄæµÄŹĒ ”£
¢ŚIµē³ŲŠŌÄÜÓÅŌ½£¬I-Ag2O µē³ŲæÉÓĆ×÷Ė®ĻĀ¶ÆĮ¦µēŌ“£¬ĘäŌĄķČēĶ¼ĖłŹ¾”£øƵē³Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£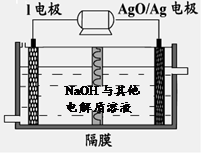
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌŚĻĀĶ¼ĖłŹ¾µÄĪļÖŹ×Ŗ»Æ¹ŲĻµÖŠ£¬AŹĒŗ£Ė®ÖŠŗ¬Įæ×ī·įø»µÄŃĪ£¬BŹĒ³£¼ūµÄĪŽÉ«ŅŗĢ壬FŌŚDÖŠČ¼ÉÕ·¢³ö²Ō°×É«»šŃę”£HæÉÓĆÓŚÖĘŌģ¹āµ¼ĻĖĪ¬£¬JŹĒŅ»ÖÖČé°×É«Äż½ŗד³Įµķ”£(²æ·ÖÉś³ÉĪļŗĶ²æ·Ö·“Ó¦Ģõ¼žĪ“ĮŠ³ö)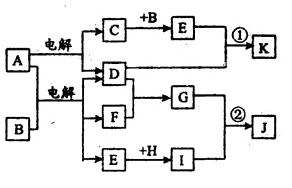
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©AµÄµē×ÓŹ½ĪŖ
ŠĪ³Éµ„ÖŹDµÄŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆ
£Ø2£©ŠĪ³ÉC”¢D”¢Fµ„ÖŹµÄŌŖĖŲ°ė¾¶Óɓ󵽊”µÄĖ³Šņ(ĢīŠ“ŌŖĖŲ·ūŗÅ)
£Ø3£©C³¤ĘŚ±©Ā¶ŌŚæÕĘųÖŠ£¬×īÖÕ²śĪļŹĒ
£Ø4£©HµÄ»ÆѧŹ½ĪŖ HŌŚøßĪĀĻĀÓėĢ¼·“Ó¦£¬Čō×ŖŅĘ4molµē×Ó²Ī¼Ó·“Ó¦µÄĢ¼ĪŖ mol”£
£Ø5£©Š“³ö·“Ó¦¢ŁµÄĄė×Ó·½³ĢŹ½
£Ø6£©Š“³ö·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠŹµŃé”°²Ł×÷ŗĶĻÖĻó”±Óė”°½įĀŪ”±¶ŌÓ¦¹ŲĻµÕżČ·µÄŹĒ
| | ²Ł×÷ŗĶĻÖĻó | ½įĀŪ |
| A | Ļņ×°ÓŠFe(NO3)2ČÜŅŗµÄŹŌ¹ÜÖŠ¼ÓČėĻ”H2SO4,ŌŚ¹ÜæŚ¹Ū²ģµ½ŗģ×ŲÉ«ĘųĢå | HNO3·Ö½āÉś³ÉĮĖNO2 |
| B | Ļņµķ·ŪČÜŅŗÖŠ¼ÓČėĻ”H2SO4£¬¼ÓČČ¼ø·ÖÖÓ£¬ĄäČ“ŗóŌŁ¼ÓČėŠĀÖĘCu(OH)2×ĒŅŗ£¬¼ÓČČ£¬Ć»ÓŠŗģÉ«³ĮµķÉś³É | µķ·ŪƻӊĖ®½ā³ÉĘĻĢŃĢĒ |
| C | Ļņ±„ŗĶNa2CO3ČÜŅŗÖŠĶØČė×ćĮæCO2£¬ČÜŅŗ±ä»ė×Ē | Īö³öĮĖNaHCO3 |
| D | ĻņĪŽĖ®ŅŅ“¼ÖŠ¼ÓČėÅØH2SO4£¬¼ÓČČÖĮ170”ę²śÉśµÄĘųĢåĶØČėĖįŠŌKMnO4ČÜŅŗ£¬ŗģÉ«ĶŹČ„ | Ź¹ČÜŅŗĶŹÉ«µÄĘųĢåŹĒŅŅĻ© |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com