
���� ��1��ʹ��������ƽ�IJ���Ϊ�������������������������Ʒ��ȡ��Ʒ��ȡ�����������㣻
��2�����ݳ�������ҩƷʱ������ҩƷ����ȷ�������
��3����������һ�����ʵ���Ũ����Һ�IJ������輰ÿ���õ����������
��4�������������������ʵ����ʵ�������Һ�������Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1��������ƽ�IJ���Ϊ�������������������������Ʒ��ȡ��Ʒ��ȡ�����������㣬����ȷ�IJ���˳���ǣ�B��C��F��A��E��D��B��
�ʴ�Ϊ��C��F��E��D��B��
��2����������ҩƷʱ������ҩƷֻȱ��������ʱ��������ҩ�ף���������������������С����ҩ��ʹʹ��������������ֽ�ϣ�
�ʴ�Ϊ��������ҩ�ף���������������������С����ҩ��ʹʹ��������������ֽ�ϣ�
��3������һ�����ʵ���Ũ����Һ�IJ������裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȣ��ܽ�ʱ������Ӧ�����ձ��У��ò��������Ͻ��裻
�ʴ�Ϊ���ձ�����������250mL����ƿ��ϴ�ӡ����ݡ�ҡ�ȣ�
��4��A����������������ϣ�BaCl2���������Ͻ��г��������³�ȡ������ƫС�����ʵ����ʵ���ƫС����Һ��Ũ��ƫ�ͣ���Aѡ��
B��ѡ�õ�����ƿ������������ˮ�������ʵ����ʵ�������Һ��������������Ӱ�죬��Һ��Ũ�Ȳ���Ӱ�죬��B��ѡ��
C������ҡ�Ⱥ�Һ���½����ּ�ˮ���̶��ߣ�������Һ�����ƫ����Һ��Ũ��ƫ�ͣ���Cѡ��
D���������ƹ����У�����ƿ�����ᵼ����Һ��ϲ����ȣ���Һ���������ƫ���ƫС����Һ��Ũ�ȿ���ƫ�ͻ�ƫ�ߣ���D��ѡ��
��ѡ��AC��
���� ���⿼��������һ�����ʵ���Ũ����Һ�����ؿ���ѧ����ʵ��������������ճ̶ȣ���ȷʵ��ԭ���ͻ��������ǽ���ؼ�����Ŀ�ѶȲ���ע��������ƽ��ʹ�÷�����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ų�������Ϊ��0.4Q1+0.05Q3��KJ | B�� | �ų�������Ϊ��0.4Q1+0.05Q2��KJ | ||
| C�� | ��H2=��H3 | D�� | ��H2����H3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
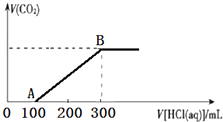 200mLij���ʵ���Ũ�ȵ�NaOH��Һ�л���ͨ��һ������CO2����ַ�Ӧ�õ�Na2CO3��NaHCO3�Ļ����Һ��������������Һ�У���εμ�2mol•L-1�����ᣬ����������������������������ϵ��ͼ��ʾ��
200mLij���ʵ���Ũ�ȵ�NaOH��Һ�л���ͨ��һ������CO2����ַ�Ӧ�õ�Na2CO3��NaHCO3�Ļ����Һ��������������Һ�У���εμ�2mol•L-1�����ᣬ����������������������������ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ԫ��R λ�����ڱ���IB �壬��ԭ������Ϊa����ԭ������Ϊa-3 ��Ԫ��λ�ڢ�B �� | |
| B�� | ��Ԫ�����ڱ��� 114 ��Ԫ�ص���һ����ͬһ��Ԫ�ص�ԭ�������� 82 | |
| C�� | ������ͬ���Ӳ�ṹ������Ԫ������ΪX2+��Y+��������������ˮ����ļ���X��Y | |
| D�� | �����ڱ��н�����ǽ����ķֽ��ߴ������ҵ����������¡���ʴ�ĺϽ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
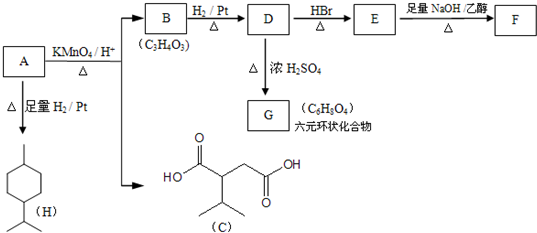
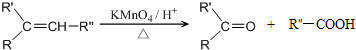
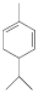 ��
��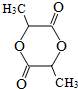 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ס��졢�� | B�� | �졢�ڡ��� | C�� | �졢�졢�� | D�� | �ס��ڡ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢܢ� | B�� | �ڢܢ� | C�� | �ڢ� | D�� | �٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
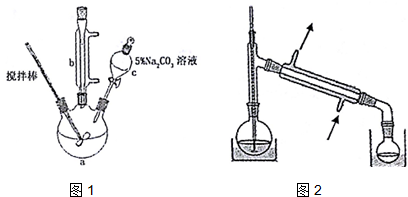
| �е� | �ܽ��� | ||
| ��ϩ�� | 141�� | ��ˮ���ܣ��������л��ܼ� | �ж� |
| �״� | 65�� | ��ˮ���ܣ��������л��ܼ� | �ӷ����ж� |
| ��ϩ����� | 80.5�� | ������ˮ���������л��ܼ� | �ӷ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 5mL | B�� | 20mL | C�� | ����5mL | D�� | ��5mL |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com