
������A��ʯ�ͻ�����һ����Ҫԭ�ϣ���A��ˮú��Ϊԭ�Ͼ�����;���ϳɻ�����D������ʽΪC3H6O3����


������������⣺
��1��д���������ʵĽṹ��ʽ��
A��____________,B��____________,C��____________,D��____________��
��2��ָ����Ӧ�ڵķ�Ӧ����________________________��
��3��д����Ӧ�۵Ļ�ѧ����ʽ____________________________________________________��
��4����Ӧ�ܵ�Ŀ����____________________________________________________________��
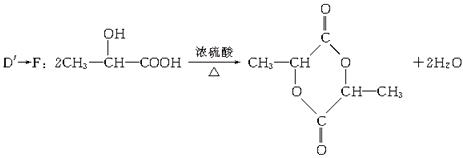
��5��������D����D��һ��ͬ���칹�壬�����緢������ţ���У��������������л���м���D����Ũ������ڵ������¼��ȣ��ȿ���������ʹ��ˮ��ɫ�Ļ�����E��C3H4O2�����ֿ���������ԭ�ӻ�״������F��C6H8O4������ֱ�д��D������E��F�Ļ�ѧ����ʽ��
D���E��_____________________________________________________________________��
D���F��____________________________________________________________________��
��1��A��CH2==CH2
B��CH3CH2CHO
C��CH2==CHCHO
D��CH2��OH��CH��OH��CHO
��2��ȡ��
��3��CH3CHBrCHO+NaOH![]() CH2==CHCHO+NaBr+H2O
CH2==CHCHO+NaBr+H2O
��4������ȩ������ֹ��Ӧ��ʱ��������ؼ�����Һ����
��5��D���E��CH3CH��OH��COOH![]() CH2==CHCOOH+H2O
CH2==CHCOOH+H2O
������CH3CHBrCHOΪͻ�ƿڣ��ɵ��Ƴ�BΪCH3CH2CHO���Ӷ��Ƴ�AΪCH2==CH2��CΪCH2==CHCHO��DΪCH2��OH��CH��OH��CHO�����ڽ�̼̼˫������ʱ���������ȩ���������������轫ȩ�����Ա�����
D����D��Ϊͬ���칹�壬�����ܷ�����ȥ��Ӧ��˵�����С�OH���ܷ����ɻ���Ӧ��˵�������ڻ����С�COOH���Ӷ��ó�D��ΪCH3CH��OH��COOH��


 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| �� |
| �� |
| �� |
| �� |
| Ũ���� |
| �� |
| Ũ���� |
| �� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������A��ʯ�ͻ�����һ����Ҫԭ�ϣ���A��ˮú��Ϊԭ�Ͼ�ͼ��ʾ;���ϳɻ�����D(����ʽΪC3H6O3)��

��ش��������⣺
(1)д���������ʵĽṹ��ʽ��A ��B ��C ��D ��
(2)ָ����Ӧ�ڵķ�Ӧ���ͣ� ��
(3)д����Ӧ�۵Ļ�ѧ����ʽ�� ��
(4)��Ӧ�ܵ�Ŀ���� ��
(5)������D����D��һ��ͬ���칹�壬�����緢������ţ���У��������������л���м���D����Ũ������ڵ������¼��ȣ��ȿ���������ʹ��ˮ��ɫ�Ļ�����E(C3H4O2)���ֿ���������ԭ�ӻ�״������F(C6H8O4)����ֱ�д��D������E��F�Ļ�ѧ����ʽ��
D���E�� ��
D���F�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����У�Ϻ��У�����������ʦ������У�����������ѧ�Ծ����������� ���ͣ������
�����12�֣�
������A��ʯ�ͻ�����һ����Ҫԭ�ϣ���A��ˮú��Ϊԭ�Ͼ�����;���ϳɻ�����D������ʽΪC3H6O3����
��֪��




��ش��������⣻
��1��д���й����ʵĽṹ��ʽ��A�� ��B�� ��C�� ��D�� ��
��2��ָ����Ӧ�ݵķ�Ӧ���� ��
��3��д����Ӧ�۵Ļ�ѧ����ʽ ��
��4����Ӧ�ܵ�Ŀ���� ��
������D����D��һ��ͬ���칹�壬�����緢������ţ���У��������������л���м���D����Ũ������ڵ������¼��ȣ��ȿ�������ʹ��ˮ��ɫ�Ļ�����E��C3H4O2�����ֿ�������ԭ�ӻ�״������F��C6H8O4����
��ֱ�д��D������E��F�Ļ�ѧ����ʽ��
��5��D����E��
��6��D����F��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ����У�Ϻ��У�����������ѧ�Ծ��������棩 ���ͣ������
�����12�֣�
������A��ʯ�ͻ�����һ����Ҫԭ�ϣ���A��ˮú��Ϊԭ�Ͼ�����;���ϳɻ�����D������ʽΪC3H6O3����
��֪��



��ش��������⣻
��1��д���й����ʵĽṹ��ʽ��A�� ��B�� ��C�� ��D�� ��
��2��ָ����Ӧ�ݵķ�Ӧ���� ��
��3��д����Ӧ�۵Ļ�ѧ����ʽ ��
��4����Ӧ�ܵ�Ŀ���� ��
������D����D��һ��ͬ���칹�壬�����緢������ţ���У��������������л���м���D����Ũ������ڵ������¼��ȣ��ȿ�������ʹ��ˮ��ɫ�Ļ�����E��C3H4O2�����ֿ�������ԭ�ӻ�״������F��C6H8O4����
��ֱ�д��D������E��F�Ļ�ѧ����ʽ��
��5��D����E��
��6��D����F��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com