
A.�٣��ڣ��ۣ��ܣ���
B.�ڣ��٣��ۣ��ܣ���
C.�ڣ��ۣ��ܣ��٣���
D.�ݣ��ܣ��ۣ��ڣ���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1998��ȫ����35)�������������ڲ�ͬ�¶��µ��ܽ��(g/100 g H2O)
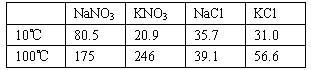
(����ʱ�ٶ����������ʱ��Ӱ����Ե��ܽ�ȣ��ڹ��˾���ʱ���ܼ���ĺ��Բ��ơ�)
(1)ȡ23.4 g NaCl��40.4 g KNO3����70.0 g H2O�������ܽ⡣��100��ʱ������50.0 g H2O��ά�ָ��¶ȣ������������壬�������þ��������(![]() )��
)��
����Һ��ȴ��10�棬����ֽᾧ���ˡ��������þ��������(![]() )��
)��
(2)��ȡ34.0 g NaNO3��29.8 g KCl��ͬ����������ʵ�顣10��ʱ�����ľ����� (д��ѧʽ)��100���10��õ��ľ�������(m��������m������)�ֱ��Ƕ���?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.1��4 B.1��5 C.2��1 D.2��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.NO��0.001 mol��L��1 B.H2O��0.002 mol��L��1
C.NH3��0.002 mol��L��1 D.O2��0.00125 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.11��1 B.9��1 C.1��11 D.1��9
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com