
³£ĪĀĻĀ£¬Ņ»ÖÖĶéĢžAŗĶŅ»ÖÖµ„Ļ©ĢžB×é³ÉµÄ»ģŗĻĘųĢ壬A»ņB·Ö×Ó×ī¶ąÖ»ŗ¬ÓŠ4øöĢ¼Ō×Ó£¬ĒŅB·Ö×ÓµÄĢ¼Ō×ÓŹż±ČA·Ö×ӵĶą”£
£Ø1£©½«1L»ģŗĻĘųĢå³ä·ÖČ¼ÉÕ£¬ŌŚĶ¬ĪĀĶ¬Ń¹ĻĀµĆµ½2.5LCO2ĘųĢ唣ŹŌĶعż¼ĘĖćĶʶĻŌ»ģŗĻĘųĢåÖŠAŗĶBĖłÓŠæÉÄܵÄ×éŗĻ¼°ĘäĢå»ż±Č£¬²¢½«½į¹ūĢīČėĻĀ±ķ£ŗ
| ×éŗĻ±ąŗÅ | AµÄ·Ö×ÓŹ½ | BµÄ·Ö×ÓŹ½ | AŗĶBµÄĢå»ż±Č£ØVA £ŗVB£© |
| ¢Ł | | | |
| ¢Ś | | | |
| ¢Ū | | | |
| ¢Ü | | | |
£Ø1£©£Ø·Ö×ÓŹ½ø÷0.5·Ö£¬Ģå»ż±Čø÷1·Ö£¬¹²8·Ö£©×éŗĻ±ąŗÅ AµÄ·Ö×ÓŹ½ BµÄ·Ö×ÓŹ½ AŗĶBµÄĢå»ż±Č£ØVA £ŗVB£© ¢Ł CH4 C3H6 1 £ŗ3 ¢Ś CH4 C4H8 1 £ŗ1 ¢Ū C2H6 C3H6 1 £ŗ1 ¢Ü C2H6 C4H8 3 £ŗ1
£Ø2£©C2H6”¢C4H8
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©Ņņ1Éż»ģŗĻĘųĢå³ä·ÖČ¼ÉÕŗóÉś³É2.5ÉżCO2£¬ĒŅB·Ö×ÓµÄĢ¼Ō×ÓŹż±ČA·Ö×ӵĶą£¬»ģŗĻĘųĢåÖ»ÄÜÓÉĢ¼Ō×ÓŹżŠ”ÓŚ2.5µÄĶéĢž£ØCH4ŗĶC2H6£©ŗĶĢ¼Ō×ÓŹż“óÓŚ2.5µÄĻ©Ģž£ØC3H6ŗĶC4H8£©×é³É£¬ĖüĆĒÓŠĖÄÖÖæÉÄܵÄ×éŗĻ£ŗCH4”¢C3H6£»CH4”¢C4H8£»C2H6”¢C3H6£»C2H6”¢C4H8£»øł¾ŻĆæŅ»ÖÖ×éŗĻÖŠĶéĢžŗĶĻ©ĢžµÄĢ¼Ō×ÓŹż¼°Č¼ÉÕŗóÉś³ÉµÄCO2Ģå»ż£¬æÉČ·¶ØAŗĶBµÄĢå»ż±Č£®Čē£ŗ £¬ŌņV£ØCH4£©£ŗV£ØC3H6£©£½1£ŗ3£¬ŅŌ“ĖĄąĶĘæÉ¢ŚCH4”¢C4H8”¢1£ŗ1¢ŪC2H6”¢C3H6”¢1£ŗ1¢ÜC2H6”¢C4H8”¢3£ŗ1£»
£¬ŌņV£ØCH4£©£ŗV£ØC3H6£©£½1£ŗ3£¬ŅŌ“ĖĄąĶĘæÉ¢ŚCH4”¢C4H8”¢1£ŗ1¢ŪC2H6”¢C3H6”¢1£ŗ1¢ÜC2H6”¢C4H8”¢3£ŗ1£»
£Ø2£©Éč1ÉżĘųĢ¬ĢžÓėŃõ³ä·ÖČ¼ÉÕŗóĢå»ż±ä»ÆĪŖ”÷VÉż£¬Ōņ
CH4+2O2 CO2+2H2O£ØĘų£© ”÷V1=0£ØÉż£©
CO2+2H2O£ØĘų£© ”÷V1=0£ØÉż£©
C2H6+ O2
O2 2CO2+3H2O£ØĘų£©”÷V2=0.5£ØÉż£©
2CO2+3H2O£ØĘų£©”÷V2=0.5£ØÉż£©
C3H6+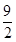 O2
O2 3CO2+3H2O£ØĘų£©”÷V3=0.5£ØÉż£©
3CO2+3H2O£ØĘų£©”÷V3=0.5£ØÉż£©
C4H8+6O2 4CO2+4H2O£ØĘų£©”÷V4=1.0£ØÉż£©
4CO2+4H2O£ØĘų£©”÷V4=1.0£ØÉż£©
ø÷ÖÖ×éŗĻµÄ1Éż»ģŗĻĘųĢåÓėŃõĘų³ä·ÖČ¼ÉÕ£¬Ģå»żŌö“óĪŖ×éŗĻ¢Ł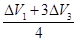 =0.375£ØÉż£©
=0.375£ØÉż£©
×éŗĻ¢Ś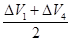 =0.5£ØÉż£©
=0.5£ØÉż£©
×éŗĻ¢Ū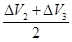 =0.5£ØÉż£©
=0.5£ØÉż£©
×éŗĻ¢Ü =0.625£ØÉż£©
=0.625£ØÉż£©
Ōņ ”Į100%=6.25%
”Į100%=6.25%
¹Ź×éŗĻ¢Ü·ūŗĻĢāŅā£¬¼“A£ŗC2H6£»B£ŗC4H8”£
æ¼µć£ŗæ¼²éÓŠ»śĪļ»ÆѧŹ½Č·¶ØµÄÓŠ¹Ų¼ĘĖć


 ¼¤»īĖ¼Ī¬ÖĒÄÜѵĮ·æĪŹ±µ¼Ń§Į·ĻµĮŠ“š°ø
¼¤»īĖ¼Ī¬ÖĒÄÜѵĮ·æĪŹ±µ¼Ń§Į·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¶Ō¼×»ł±½¼×ĖįŅŅõ„( )ŹĒÓĆÓŚŗĻ³ÉŅ©ĪļµÄÖŠ¼äĢ唣Ēėøł¾ŻĻĀĮŠ×Ŗ»Æ¹ŲĻµ»Ų“šÓŠ¹ŲĪŹĢā
)ŹĒÓĆÓŚŗĻ³ÉŅ©ĪļµÄÖŠ¼äĢ唣Ēėøł¾ŻĻĀĮŠ×Ŗ»Æ¹ŲĻµ»Ų“šÓŠ¹ŲĪŹĢā
(1)DÖŠŗ¬ÓŠ¹ŁÄÜĶŵÄĆū³ĘŹĒ________£¬A£EµÄ·“Ó¦ĄąŠĶĪŖ______”£
(2)GµÄ½į¹¹¼ņŹ½ĪŖ_______”£
(3)Š“³ö1ÖÖŹōÓŚõ„ĄąĒŅ±½»·ÉĻÖ»ÓŠŅ»øöČ”“ś»łµÄC8H8O2µÄĶ¬·ÖŅģ¹¹Ģå ”£
(4)¶”Ļć·Ó( )ŹĒ¶Ō¼×»ł±½¼×ĖįŅŅõ„µÄĶ¬·ÖŅģ¹¹Ģ壬ĻĀĮŠĪļÖŹÓėĘäÄÜ·¢Éś·“Ó¦µÄŹĒ________(ĢīŠņŗÅ)”£
)ŹĒ¶Ō¼×»ł±½¼×ĖįŅŅõ„µÄĶ¬·ÖŅģ¹¹Ģ壬ĻĀĮŠĪļÖŹÓėĘäÄÜ·¢Éś·“Ó¦µÄŹĒ________(ĢīŠņŗÅ)”£
| A£®NaOHČÜŅŗ | B£®NaHCO3ČÜŅŗ | C£®KMnO4/H+ | D£®FeCl3ČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓŠŌö³¤Ģ¼Į“ŹĒÓŠ»śŗĻ³ÉÖŠ·Ē³£ÖŲŅŖµÄ·“Ó¦”£ĄżČē£ŗ
·“Ó¦¢Ł
ÓĆ ĶعżŅŌĻĀĀ·ĻßæÉŗĻ³É(¢ņ)£ŗ
ĶعżŅŌĻĀĀ·ĻßæÉŗĻ³É(¢ņ)£ŗ
£Ø1£©£Ø¢ń£©µÄ·Ö×ÓŹ½ĪŖ £»1moløĆĪļÖŹĶźČ«Č¼ÉÕŠčŅŖĻūŗÄ mol O2.”£
£Ø2£©£Ø¢ņ)Óė×ćĮæµÄČČNaOHČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø¢ó£©µÄ½į¹¹¼ņŹ½ĪŖ £» ŌŚÉś³É£Ø¢ó£©Ź±£¬»¹ÄܵƵ½ĮķŅ»ÖÖø±²śĪļC6H8O4£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £¬øĆ·“Ó¦µÄ·“Ó¦ĄąŠĶŹĒ ”£
ŌŚÉś³É£Ø¢ó£©Ź±£¬»¹ÄܵƵ½ĮķŅ»ÖÖø±²śĪļC6H8O4£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £¬øĆ·“Ó¦µÄ·“Ó¦ĄąŠĶŹĒ ”£
£Ø4£©¶Ō¶žĀȱ½ Ņ²ÄÜÓėÓŠ»śĪļ(¢ń) (¹żĮæ)·¢ÉśĄąĖĘ·“Ó¦¢ŁµÄĻµĮŠ·“Ó¦£¬ĘäÉś³ÉÓŠ»śĪļµÄ½į¹¹¼ņŹ½ĪŖ ”£
Ņ²ÄÜÓėÓŠ»śĪļ(¢ń) (¹żĮæ)·¢ÉśĄąĖĘ·“Ó¦¢ŁµÄĻµĮŠ·“Ó¦£¬ĘäÉś³ÉÓŠ»śĪļµÄ½į¹¹¼ņŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
æɽµ½ā¾ŪŗĻĪļ¾ŪĢ¼Ėįõ„µÄŗĻ³ÉĀ·ĻßČēĻĀ£¬ĘäÖʱø¹ż³ĢÖŠ»¹æÉÖʵĆøß·Ö×Ó²ÄĮĻF”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©·“Ó¦¢ŁµÄ·“Ó¦ĄąŠĶĪŖ ”£
£Ø2£©Š“³öDµÄ½į¹¹¼ņĪä ”£
£Ø3£©E£ØC5H8O2£©·Ö×ÓÖŠĖłŗ¬¹ŁÄÜĶŵÄĆū³Ę ”£
£Ø4£©Š“³öŌŚŅ»¶ØĢõ¼žĻĀDÓė ·“Ó¦ŗĻ³É¾ŪĢ¼õ„µÄ»Æѧ·½³ĢŹ½ ”£
·“Ó¦ŗĻ³É¾ŪĢ¼õ„µÄ»Æѧ·½³ĢŹ½ ”£
£Ø5£©Š“³öŅ»ÖÖĶ¬Ź±·ūŗĻĻĀĮŠĢõ¼žµÄEµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ ”£
¢ŁÓėE¾ßÓŠĻąĶ¬¹ŁÄÜĶÅ
¢ŚÄÜ·¢ÉśŅų¾µ·“Ó¦
¢ŪHŗĖ“Ź²ÕńĘ×ÖŠÓŠĖÄÖÖ²»Ķ¬»·¾³µÄĒāŌ×Ó£¬±ČĄż·Ö±šĪŖ1:2:2:3
£Ø6£©ŅĄ¾ŻĀĢÉ«»ÆѧŌĄķ£¬Ō×ӵĥūÓĆĀŹĪŖ100%”£ŅŌ±ūČ²”¢¼×“¼ĪŖŌĮĻæÉŗĻ³ÉF£¬ĒėÉč¼ĘŗĻ³ÉĀ·ĻߣØĪŽ»śŹŌ¼ĮČĪŃ”£© ”£ŗĻ³ÉĀ·ĻßĮ÷³ĢĶ¼Ź¾ĄżČēĻĀ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓŠĮ½ÖÖĘųĢ¬ĢžµÄ»ģŗĻĪļ”£ŅŃÖŖ£ŗĖüĆĒ¶¼ÄÜŹ¹äåĖ®ĶŹÉ«”£ĒŅ·Ö×ÓÖŠĢ¼Ō×ÓŹż¾łŠ”ÓŚ5£»1Ģå»żøĆ»ģŗĻĘųĢåĶźČ«Č¼ÉÕŗó£¬æɵƵ½3.6Ģå»ż¶žŃõ»ÆĢ¼ŗĶ3Ģå»żĖ®ÕōĘų(ĘųĢåĢå»ż¾łŌŚĶ¬ĪĀĶ¬Ń¹ĻĀ²ā¶Ø)
(1)»ģŗĻĪļÖŠĮ½ÖÖĢžµÄĄą±šæÉÄÜŹĒ________”£
| A£®Ķ飬Ļ© | B£®Ļ©£¬Ļ© | C£®Ļ©£¬Č² | D£®Č²£¬Č² |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¹²¾Ū·ØæÉøĽųÓŠ»śøß·Ö×Ó»ÆŗĻĪļµÄŠŌÖŹ£¬øß·Ö×Ó¾ŪŗĻĪļPµÄŗĻ³ÉĀ·ĻßČēĻĀ£ŗ

£Ø1£©AµÄ½į¹¹Ź½ĪŖ________________ £¬CµÄĆū³ĘĪŖ
£Ø2£©DÖŠŗ¬ÓŠµÄ¹ŁÄÜĶŵÄĆū³Ę
£Ø3£©ÓÉF¾¢Ł”«¢ŪŗĻ³ÉI£¬FæÉŅŌŹ¹äåĖ®ĶŹÉ«£®·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½ŹĒ ”£
·“Ó¦¢ŚµÄ·“Ó¦ĄąŠĶŹĒ ·“Ó¦¢ŪµÄ·“Ó¦ŹŌ¼ĮŹĒ ”£
£Ø4£©ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ ”£
a£®CæÉÓėĖ®ČĪŅā±ČĄż»ģŗĻ
b£®AÓė1,3-¶”¶žĻ©»„ĪŖĶ¬ĻµĪļ
c£®ÓÉIÉś³ÉMŹ±£¬1mol I×ī¶ąĻūŗÄ3molNaOH
d£®N²»“ęŌŚĖ³·“Ņģ¹¹Ģå
£Ø5£©Š“³öÓÉDÉś³ÉEµÄ»Æѧ·½³ĢŹ½ ”£
£Ø6£©EÓėNµČĪļÖŹµÄĮæ·¢Éś¹²¾Ū·“Ӧɜ³ÉP£¬ŌņPµÄ½į¹¹¼ņŹ½ĪŖ ”£
£Ø7£©EÓŠ¶ąÖÖĶ¬·ÖŅģ¹¹Ģ壬Š“³ö·ūŗĻĻĀĮŠĢõ¼žµÄĖłÓŠĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ ”£
a£®±½»·ÉĻÓŠĮ½øöČ”“ś»ł
b£®ÄÜŹ¹FeCl3ŅŗĻŌ×ĻÉ«
c£®1moløĆÓŠ»śĪļÓėÅØäåĖ®·“Ó¦Ź±ÄÜĻūŗÄ4molBr2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻÖÄā·ÖĄėŅŅĖįŅŅõ„”¢ŅŅĖį”¢ŅŅ“¼µÄ»ģŗĻĪļ£¬ČēĶ¼ŹĒ·ÖĄė²Ł×÷²½ÖčĮ÷³ĢĶ¼”£ĒėĢīČėŹŹµ±µÄŹŌ¼Į”¢·ÖĄė·½·ØŅŌ¼°Ėł·ÖĄėµÄÓŠ¹ŲĪļÖŹµÄĆū³Ę”£
(1)a________£¬b________£»
(2)¢Ł________£¬¢Ś________£¬¢Ū________£»
(3)A________£¬B_________£»C________£»D_______£¬E_______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĶŃĖ®»·»ÆŹĒŗĻ³ÉÉśĪļ¼īĄąĢģČ»²śĪļµÄÖŲŅŖ²½Öč£¬Ä³ÉśĪļ¼īŗĻ³ÉĀ·ĻßČēĻĀ£ŗ
£Ø1£©»ÆŗĻĪļ¢óµÄ»ÆѧŹ½ĪŖ ”£
£Ø2£©»ÆŗĻĪļAµÄ½į¹¹¼ņŹ½ĪŖ ”££Ø3£©·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ ”£
| A£®»ÆŗĻĪļ¢ņÄÜ·¢ÉśŅų¾µ·“Ó¦ | B£®»ÆŗĻĪļ¢ń”«¢õ¾łŹōÓŚ·¼ĻćĢž |
| C£®·“Ó¦¢ŪŹōÓŚõ„»Æ·“Ó¦ | D£®»ÆŗĻĪļ¢ņÄÜÓė4 molH2·¢Éś¼Ó³É·“Ó¦ |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓŠ»śĪļA(C12H15ClO3)ĪŖŗ¬¶ąÖÖ¹ŁÄÜĶŵķ¼Ļć×å»ÆŗĻĪļ£¬Ęä½į¹¹¼ņŹ½ČēĶ¼(ĘäÖŠŠéĻßæņÄŚĪŖĪ“ÖŖ²æ·ÖµÄ½į¹¹)ĖłŹ¾£¬AæÉ·¢ÉśČēĻĀ×Ŗ»Æ(ĘäÖŠ²æ·Ö²śĪļŅŃĀŌČ„)”£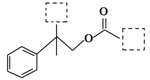
ŅŃÖŖ£ŗDÄÜŹ¹äåµÄCCl4ČÜŅŗĶŹÉ«£»B²»ÄÜÓėFeCl3ČÜŅŗ·¢ÉśĻŌÉ«·“Ó¦£¬1mol B×ī¶ąÄÜÓė2mol½šŹōÄĘ·“Ó¦£»CµÄ“ß»ÆŃõ»Æ²śĪļ²»ÄÜ·¢ÉśŅų¾µ·“Ó¦”£»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©Š“³öCÖŠ¹ŁÄÜĶŵÄĆū³Ę _”£
£Ø2£©AµÄ½į¹¹¼ņŹ½(Š“³öŅ»ÖÖ)£ŗ _”£
£Ø3£©Š“³ö·“Ó¦ĄąŠĶ£ŗC”śD £»B”śE ”£
£Ø4£©Š“³öEÓėŠĀÖĘCu(OH)2·“Ó¦µÄ»Æѧ·½³ĢŹ½ _”£
£Ø5£©EÓŠ¶ąÖÖĶ¬·ÖŅģ¹¹Ģ壬Āś×ćĻĀĮŠĢõ¼žµÄĶ¬·ÖŅģ¹¹ĢåÓŠ ÖÖ£¬Š“³öĘäÖŠČĪŅāŅ»ÖÖĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ ”£
¢Łŗ¬ÓŠ±½»·ĒŅ±½»·ÉĻÖ»ÓŠŅ»øöČ”“ś»ł£»¢Ś·Ö×Ó½į¹¹ÖŠŗ¬ÓŠ¼×»ł£»¢ŪÄÜ·¢ÉśĖ®½ā·“Ó¦”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com