
(10��) ��ѧʵ���Ҳ����ķ�Һ�к��д�������Ⱦ���������ʣ�Ϊ�˱�����������Щ��Һ���뾭����������ŷš�ij��ѧʵ���Ҳ����ķ�Һ�к������ֽ������ӣ�Fe3+��Cu2+����ѧС�����������ͼ��ʾ�ķ����Է�Һ���д������Ի��ս���������������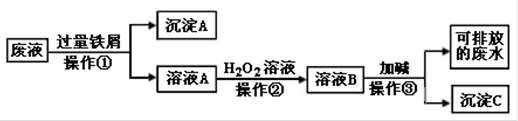
��1����Һ�������ٵõ��ij���A�к��еĽ��������� ��
��2��д�������ٵķ�Ӧ���ӷ���ʽ �� ��
��3���ڲ������й۲쵽��ʵ�������� ��
��4���������з�����Ҫ��Ӧ�����ӷ���ʽΪ ��
��1��Fe ��Cu ��2�� 2Fe3+ + Fe = 3Fe2+ ��Cu2+ + Fe =" Cu" + Fe2+
��3����Һ��dz��ɫ��Ϊ��ɫ ��4�� Fe3+ + 3OH�� = Fe(OH)3��
�����������������Fe3+��Cu2+�ķ�Һ�У����������м��������Ӧ ��2Fe3+ + Fe = 3Fe2+ ��Cu2+ + Fe =" Cu" + Fe2+�����˺õ�����A�����к���ͭ�����ĵ��ʣ����õ���Һ��ҺA�����к���Fe2+������H2O2��Һ��Fe2+��������Fe3+����Һ��ɫ��dz��ɫ��Ϊ��ɫ�������Һ�мӼ������Ӧ��Fe3+ + 3OH�� = Fe(OH)3�� ���õ�Fe(OH)3������
���㣺��Һ���մ�����Fe�Ļ�ԭ�Ժ�Fe3+�������ԡ�
����������ͨ����Һ���մ������̿���ѧ����Fe�Ļ�ԭ�Ժ�Fe3+�������Ե����գ�����ʱ��Ҫ��ÿһ���Ļ��������û�ѧ��Ӧ��ѧԭ�����ƶ�������Ƶ�ԭ���ƶ���һ����Ƶ���ͼ����Ҫѧ��������������֮��Ļ�ѧ��Ӧ������һ����Ҫ�μ�˫��ˮ��һ����ɫ����������


 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)��ѧ��ȤС���ijƷ��������Ħ�����ɷּ��京������̽����
�������ϣ�������Ħ������̼��ơ�����������ɣ������������ɷ���������ʱ���������ɡ�
��������Ʒ��̼��ƵĶ����ⶨ��
������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬�ⶨC�����ɵ�BaCO3������������ȷ��̼��Ƶ�����������
ʵ�鲽�裺
�� ����ͼ��ʾ���Ӻ�װ�ã��ȼ��װ�õ������ԣ�
�� ȷ��ȡ������Ʒ������4.00g�����ڸ������м�����Ӧ�Լ���
�� ���ɼмף�����ͨ�������Ȼ�����C��
�� ��B�ķ�Һ©���μ����ᷴӦ��ͬʱ�ٴγ�������ͨ�������
�� ��B�в��ٲ��������ֹͣ�μ����
�� ��C�г������ˡ�ϴ�ӡ������BaCO3��
�� ����BaCO3������Ϊ1.97g��
����ʵ����̻ش��������⣺
��1�������ͨ�������������У�______________ __��___ __________��
��2��������м������ϴ�ɾ��ķ����� ��
��3����ʵ��ⶨ�õ�����Ʒ��̼��Ƶ���������Ϊ ������ȡ���д�ʩ���Բⶨ�����ȷ��û��Ӱ�����_____________�����ţ���
a.ʡ�Բ���� b.�μ������һЩ
c. ��B��C֮������ʢ�б���NaHCO3��Һ��ϴ��װ��
d. ���ʵ�飬ȡƽ��ֵ����
��4����ͬѧ��Ϊ���زⶨC�����ɵ�BaCO3������ֻҪ�ⶨװ��C������CO2ǰ��������һ������ȷ�ⶨ̼��Ƶ���������������˵���Ƿ���Բ���Ҫ˵���� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��³����1��2��Ԫ��������������ϰ ���ͣ�ʵ����
(10��)��ѧ��һ����ʵ��Ϊ��������Ȼ��ѧ����ѧʵ���ڻ�ѧѧϰ�о��м�����Ҫ�����á�
(1)���й���ʵ���������ȷ����______��
| A��ȼ�ŵľƾ��Ʋ�������ʧ��Ӧ������ʪ������ |
| B��������Һ��pHʱ��Ӧ�Ƚ�pH��ֽ��ʪ |
| C������������Һʱ����������Ͳ�ڼ���һ�������ˮ�����ڽ�������������Ũ���� |
| D��������Ũ����Һմ��Ƥ���ϣ�Ҫ�����ô���ˮ��ϴ��Ȼ��Ϳ��������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015���㽭����ڶ���ѧ��һ�ڶ��Σ�12�£��¿���ѧ�Ծ��������棩 ���ͣ������
(10��) ��ѧʵ���Ҳ����ķ�Һ�к��д�������Ⱦ���������ʣ�Ϊ�˱�����������Щ��Һ���뾭����������ŷš�ij��ѧʵ���Ҳ����ķ�Һ�к������ֽ������ӣ�Fe3+��Cu2+����ѧС�����������ͼ��ʾ�ķ����Է�Һ���д������Ի��ս���������������
��1����Һ�������ٵõ��ij���A�к��еĽ��������� ��
��2��д�������ٵķ�Ӧ���ӷ���ʽ �� ��
��3���ڲ������й۲쵽��ʵ�������� ��
��4���������з�����Ҫ��Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��³����1��2��Ԫ��������������ϰ ���ͣ�ʵ����
(10��)��ѧ��һ����ʵ��Ϊ��������Ȼ��ѧ����ѧʵ���ڻ�ѧѧϰ�о��м�����Ҫ�����á�
(1)���й���ʵ���������ȷ����______��
A��ȼ�ŵľƾ��Ʋ�������ʧ��Ӧ������ʪ������
B��������Һ��pHʱ��Ӧ�Ƚ�pH��ֽ��ʪ
C������������Һʱ����������Ͳ�ڼ���һ�������ˮ�����ڽ�������������Ũ����
D��������Ũ����Һմ��Ƥ���ϣ�Ҫ�����ô���ˮ��ϴ��Ȼ��Ϳ��������Һ
(2)Ҫ����500 mL 0.2 mol��L��1��FeSO4��Һ��ʵ����������У�������ƽ�ϳ�ȡa g�̷�(FeSO4��7H2O)�����������ձ��У�����������ˮʹ����ȫ�ܽ⣻�ڽ�������Һ�ز�����ע��500 mL����ƿ�У��ۼ���������ƿ�м�ˮ��Һ���̶���1 cm��2 cm�������ý�ͷ�ιܼ�����ˮ��Һ�尼Һ����ʹ���̶������У���������ˮϴ���ձ��Ͳ�����2�Ρ�3�Σ�ÿ��ϴ��Һ��ת������ƿ���ݽ�����ƿ���������ҡ�ȡ���д���пհף�
��a g�̷���ʵ������Ϊ________ g��
�����������������ȷ˳��Ϊ__________________��
��������ʱ��©����ܣ���ʹ������Һ��Ũ��________(�ƫ�ߡ���ƫ�͡�����Ӱ�족)��
����������ˮʱ���������̶��ߣ�����������________________�������������ʱ����Һ��������ƿ�⣬����������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧҵˮƽ����ģ�⻯ѧ�Ծ� ���ͣ�ʵ����
(10��)��ѧ��ȤС���ijƷ��������Ħ�����ɷּ��京������̽����
�������ϣ�������Ħ������̼��ơ�����������ɣ������������ɷ���������ʱ���������ɡ�
��������Ʒ��̼��ƵĶ����ⶨ��
������ͼ��ʾװ�ã�ͼ�мг�������ȥ������ʵ�飬�ⶨC�����ɵ�BaCO3������������ȷ��̼��Ƶ�����������

ʵ�鲽�裺
�� ����ͼ��ʾ���Ӻ�װ�ã��ȼ��װ�õ������ԣ�
�� ȷ��ȡ������Ʒ������4.00g�����ڸ������м�����Ӧ�Լ���
�� ���ɼмף�����ͨ�������Ȼ�����C��
�� ��B�ķ�Һ©���μ����ᷴӦ��ͬʱ�ٴγ�������ͨ�������
�� ��B�в��ٲ��������ֹͣ�μ����
�� ��C�г������ˡ�ϴ�ӡ������BaCO3��
�� ����BaCO3������Ϊ1.97g��
����ʵ����̻ش��������⣺
��1�������ͨ�������������У�______________ __��___ __________��
��2��������м������ϴ�ɾ��ķ����� ��
��3����ʵ��ⶨ�õ�����Ʒ��̼��Ƶ���������Ϊ ������ȡ���д�ʩ���Բⶨ�����ȷ��û��Ӱ�����_____________�����ţ���
a.ʡ�Բ���� b.�μ������һЩ
c. ��B��C֮������ʢ�б���NaHCO3��Һ��ϴ��װ��
d. ���ʵ�飬ȡƽ��ֵ����
��4����ͬѧ��Ϊ���زⶨC�����ɵ�BaCO3������ֻҪ�ⶨװ��C������CO2ǰ��������һ������ȷ�ⶨ̼��Ƶ���������������˵���Ƿ���Բ���Ҫ˵���� �� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com