
����Ŀ����ҵ�����÷�������(��Ҫ�ɷ�ΪNi��������һ������Zn��Fe��SiO2��CaO��)�Ʊ�������������������£�

��1����д��һ������ߡ���������ʵĴ�ʩ��___________________________________������I�ijɷ���CaSO4��____________________(�ѧʽ)��
��2������ʱ�����Ʋ�ͬ���������Եõ���ͬ������II����֪����II�ijɷ����¶ȡ�pH�Ĺ�ϵ��ͼ��ʾ��

���������¶�40�桢pH=8��������II����Ҫ�ɷ�Ϊ___________(�ѧʽ)��
���������¶�80�桢pH=2���ɵõ���������[Na2Fe6(SO4)4(OH)12]����(ͼ����Ӱ����)��д�����ɻ������Ƶ����ӷ���ʽ��___________________________________________��
��3����֪����������100 mL��Һ��c(Ca2+)=0.01mol��L-1������100 mL NH4F��Һ��ʹCa2+ǡ�ó�����ȫ����Һ��c(Ca2+)=1��10-5 mol��L-1��������c(NH4F)=_______mol��L-1��[��֪Ksp(CaF2)=5.29��10-9]
��4�������л���ȡ����������________________________��
���𰸡��ѷ����������顢�ʵ����ȣ��ʵ��������Ũ�Ȼ�����SiO2FeOOH2Na++3ClO-+6Fe2++4SO42-+9H2O=Na2Fe6(SO4)4(OH)12��+3Cl-+6H+6.6��10-2��ȥ��Һ�е�Zn2+
��������
��������(��Ҫ�ɷ�ΪNi��������һ������Zn��Fe��SiO2��CaO��)���������ܽ���SiO2�������ᷴӦ�����˵õ���Һ�к���NiSO4��FeSO4��ZnSO4��CaSO4������������������IΪSiO2��CaSO4��������Һ�м��������������Fe2+����ΪFe3+��ͬʱ����pH��ʹFe3+ת��ΪFe(OH)3��������������IIΪ��Ԫ�صij�������Һ�к���NiSO4��CaSO4������Һ�м���NH4F����ȥCa2+���������ټ��л���ȡ����ȥ��Һ�е�Zn2+��������Һ�м�����NH4��2C2O4���õ��������������ٹ��ˡ�ϴ�ӡ�����ò�����������
(1)����Ӱ�췴Ӧ���ʵ����ش���������I��ҪΪ�����������SiO2������CaSO4��
(2)����ͼ�����֪�����ٿ����¶�40�桢pH=8��������2����Ҫ�ɷ�ΪFeOOH��
��Na2Fe6(SO4)4(OH)12����Ԫ�ػ��ϼ�Ϊ+3�ۣ���֪ClO-��e2+����ΪFe3+������������ԭ��Ӧ���ɿ�д�����ɻ������Ƶ����ӷ���ʽ��
(3)���ݷ�ӦʽCa2++2F-=CaF2��������Ca2+����0.002molNH4F������Ksp(CaF2)=c(Ca2+)c2(F-)=5.29��10-9������Ca2+����Һ��c(F-)=![]() ��
��
(4)��������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��
��1������Ӱ�컯ѧ��Ӧ���ʵ����ؿ�֪����߽����ʣ��ɰѷ�������������ʵ����ȡ��ʵ���������Ũ�ȡ�����ȣ�����I�ijɷ���CaSO4��SiO2��
��ˣ�������ȷ�������ѷ����������顢�ʵ����ȣ��ʵ��������Ũ�Ȼ�������SiO2��
��2������ͼ�����֪�����ٿ����¶�40�桢pH=8��������2����Ҫ�ɷ�ΪFeOOH��
��ˣ�������ȷ������FeOOH��
���������¶�80�桢pH=2���ɵõ���������[Na2Fe6(SO4)4(OH)12]����������������ԭ��Ӧ����д�����ɻ������Ƶ����ӷ���ʽΪ��2Na++3ClO-+6Fe2++4SO42-+9H2O=Na2Fe6(SO4)4(OH)12��+3Cl-+6H+��
��ˣ�������ȷ������2Na++3ClO-+6Fe2++4SO42-+9H2O=Na2Fe6(SO4)4(OH)12��+3Cl-+6H+��
��3�����ݷ�ӦʽCa2++2F-=CaF2��������Ca2+����0.002molNH4F������Ksp(CaF2)=c(Ca2+)c2(F-)=5.29��10-9������Ca2+����Һ��c(F-)=![]() �������c(NH4F)=cmol/L����
�������c(NH4F)=cmol/L����![]() =
=![]() �����c=6.6��10-2��
�����c=6.6��10-2��
��ˣ�������ȷ������6.6��10-2��
��4����������ͼ�������л���ȡ���������dz�ȥ��Һ�е�Zn2+��
��ˣ�������ȷ��������ȥ��Һ�е�Zn2+��


 ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڸ�ʵ��װ�ü���ʹ�õ������У���ȷ���ǣ�����װ��δ�������� ��
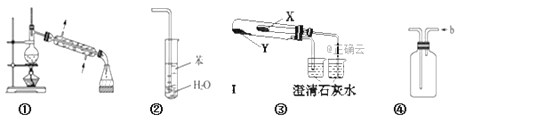
A. װ�âٳ����ڷ��뻥�����ܵ�Һ������
B. װ�âڿ���������NH3������ֹ����
C. ����װ�â���֤KHCO3��K2CO3�����ȶ��ԣ�X��Ӧ�ŵ�������KHCO3
D. װ�â� b�ڽ������ռ�Cl2��NO������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��CO��Ϊ�ȵ�����ķ��Ӻ����ӷֱ�Ϊ______��______��
(2)�����г�����һЩԭ�ӵ�2p�ܼ���3d�ܼ��е����Ų�����������ж���ЩΥ��������ԭ��__________����ЩΥ���˺��ع���__________��
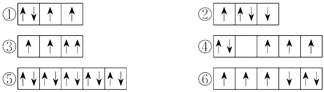
(3)ijԪ�صļ���̬(���ȶ�״̬)ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s13p33d2�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ__________��������������Ӧˮ����Ļ�ѧʽ��__________��
(4)�����ж����ԭ�ӵ�ԭ�ӹ������������ɵ͵���˳�����С�
��2s������3d������4s������3s������4p������3p
��������ɵ͵�������˳����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ۺ���������һ������Ч�����߷������������������������弰����Ϊԭ�ϴ���������������������ˮ�⡢�ۺϳɲ�Ʒ��ʵ����ģ�������������£�
![]()
��֪Fe3����ˮ�������������Fe3����3H2O===Fe��OH��3��3H����Ϊ�˷�ֹFe3��ˮ����������ᡣ
��1������ԭ������2.50 moL��L��1������������Һʱ�õ��Ķ���������_________________��
��д�����������е����ӷ���ʽ��________��
��2���ۺϿ���ʵ��Ͷ��������������������ʵ���֮��Ϊ1/1.25������ѣ���������������ֵ�Զ࣬�����ܹ����ԭ����__________________________________��
��3����������Һˮ����Եõ�һϵ�о��о�ˮ���õļ�ʽ��������xFe2O3��ySO3��zH2O�����ֲ����������ⶨx��y��z��ֵ��
�ٲⶨʱ������Լ���______����ѡ����ţ���
A��NaOH B. Ba��OH��2
C��BaCl2D��FeSO4
����Ҫ�ⶨ________��__________����������д������Ļ�ѧʽ����
��4��ѡ���ⶨ����������Ļ����������������Ⱥ�˳���г��� ______________������ţ���
�ٹ��ˡ�ϴ�ӡ����������ᾧ������ȡ����Һ�� ����ȴ���������ݺ�ɻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��C��N��S�������ﳣ�����һЩ�������⣬���й����������о��ø��ֻ�ѧ������������Щ���ʶԻ�����Ӱ�졣
��1��Ŀǰ��ҵ����һ�ַ�������CO��H2��230�棬����������ת�����ɼ״�������ˮ������ͼһ��ʾ��ѹ������0.5molCO2��1.5molH2ת���ʴ�80��ʱ�������仯ʾ��ͼ��д���÷�Ӧ���Ȼ�ѧ����ʽ__________________________________________��
��2�����������η������������е�SO2�����������£�������ͨ�루NH4)2SO3��Һ�У������ҺpH�뺬��������ʵ��������ı仯��ϵ��ͼ����ʾ��
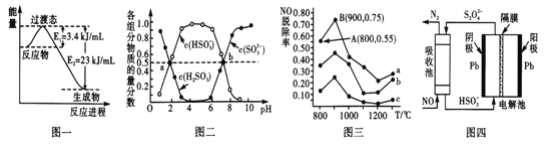
��д��a��ʱn(HSO3-)��n(H2SO3)=_____��b��ʱ��ҺpH=7����n(NH4+)��n(HSO3-)=_____��
��3����������ȥ��NO��һ�������£���NH3����NO��Ⱦ���䷴Ӧԭ��Ϊ4NH3+6NO![]() 5N2+ 6H2O����ͬ�¶������£�n(NH3):n(NO)�����ʵ���֮�ȷֱ�Ϊ4��l��3��l��1��3ʱ���õ�NO�ѳ���������ͼ����ʾ��
5N2+ 6H2O����ͬ�¶������£�n(NH3):n(NO)�����ʵ���֮�ȷֱ�Ϊ4��l��3��l��1��3ʱ���õ�NO�ѳ���������ͼ����ʾ��
�� ��д��N2�ĵ���ʽ________��
�� ����c��ӦNH3��NO�����ʵ���֮����______��
�� ����a��NO����ʼŨ��Ϊ6��10-4mg/m3����A�㵽B�㾭��0.8s����ʱ�����NO���ѳ�����Ϊ_____mg/(m3��s)��
��4����ӵ绯ѧ���ɳ�NO����ԭ����ͼ����ʾ��д�����������ĵ缫��Ӧʽ����������Һ������,����HSO3��������S2O42����:____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A(C3H6)�ǻ����л�����ԭ�ϡ���A�Ʊ��ۺ���C��![]() �ĺϳ�·��(���ַ�Ӧ������ȥ)��ͼ��ʾ��
�ĺϳ�·��(���ַ�Ӧ������ȥ)��ͼ��ʾ��
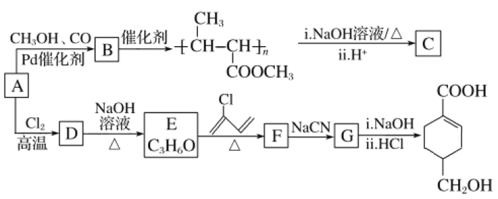
��֪��![]() ����
����![]()
![]() ��R��C��N
��R��C��N![]() R��COOH
R��COOH
�ش��������⣺
��1��A��������______________��B���еĹ����ŵ�������________________(д����)��
��2��C�Ľṹ��ʽΪ________________��D��E�ķ�Ӧ����Ϊ_____________��
��3��E��F�Ļ�ѧ����ʽΪ_____________________________________________________��
��4��![]() �������________��ԭ�ӹ�ƽ�档
�������________��ԭ�ӹ�ƽ�档
��5��B��ͬ���칹���У���B������ͬ�Ĺ��������ܷ���������Ӧ�Ĺ���________�֣����к˴Ź�������Ϊ3��壬�ҷ����֮��Ϊ6��1��1����_____________________(д�ṹ��ʽ)��
��6����������Ϣ������ϩ��HBrΪ��ʼԭ���Ʊ����ᣬ��ƺϳ�·��(�����Լ���ѡ) ______���ϳ�·������ͼʾ����CH3CHO![]() CH3COOH
CH3COOH![]() CH3COOCH2CH3
CH3COOCH2CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ���������������ѧ������������мס��Ҿ�Ϊ���ʣ����ǵ�ת����ϵ��ͼ��ʾ(ijЩ�����Ͳ��ֲ�������ȥ)������˵������ȷ���ǣ�
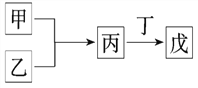
A. �����������ᷴӦ������NaOH��Һ��Ӧ�����������������������
B. ����������ϲ������̣��ұ�Ϊ18���ӷ��ӣ���Ϊ10���ӷ��ӣ����ҵ�ˮ��Һ���ܾ���Ư������
C. ����Ϊ��������ԭ�Ӱ뾶��������Ԫ�صĵ��ʣ�����Ϊ����ֻ��ΪNa2O2
D. ���ס������캬��ͬһ��Ԫ�أ������������У���Ԫ�صĻ��ϼ��ɵ͵��ߵ�˳�����Ϊ��<��<��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ���������������ѧ��ѧ������������мס��Ҿ�Ϊ���ʣ����ǵ�ת����ϵ��ͼ��ʾ(ijЩ�����Ͳ��ֲ�������ȥ)������˵������ȷ����
(����)��
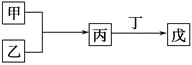
A. ����ɼס��ҵ�Ԫ��λ��ͬһ���ڣ����������һ������
B. ����ɼס��ҵ�Ԫ��λ��ͬһ���壬���������һ������
C. ����Ϊ��������ԭ�Ӱ뾶��������Ԫ���γɵĵ��ʣ�����Ϊ����ֻ��ΪNa2O2
D. ��������������ɰ��̣��ұ�Ϊ18���ӷ��ӣ���Ϊ10���ӷ��ӣ����ҵ�ˮ��Һ���ܾ���Ư������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�ֶ����ڽ���Ԫ�غ�һ�ַǽ���Ԫ����ɵĻ�����X����ˮ�������ֽⷴӦ��ijУ��ȤС������ͼװ��(�г�װ����ȥ)�������̽��ʵ�顣
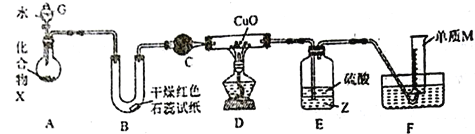
(1)����G��������_____________________��B�к�ɫʯ����ֽ����������M�ĵ���ʽΪ___________________��
(2)������X�к���ɵ���M��Ԫ����������Ϊ16.9%��д��X��ˮ��Ӧ�Ļ�ѧ����ʽ��_____________________________��
(3)C�е��Լ�����Ϊ___________________________��
(4)ʵ��ʱ��װ��D��Ӳ�ʲ������ڵ�����Ϊ_________________________��
(5)�b��E���Լ�ZΪ___________(�ѧʽ)��װ��E��������_________________________��
(6)����ͨ��E��F��װ�ã������ʵ�鷽��֤��D�з����˷�Ӧ(��ͨ���۲�D�й�����ɫ�����仯)��__________________________________________________��
(7)��װ��A�й�����Ʒ��������(���ʲ����뷴Ӧ)��ijͬѧͨ���ⶨF�е���M�ڱ�״���µ����������Ʒ����������ȷ��������Ʒ��X�������������жϸ÷����Ƿ���У���˵��ԭ��__________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com