
£Ø7·Ö£©Ä³ĪĀ¶ČĻĀ1ÉżĆܱÕČŻĘ÷ÖŠ¼ÓČė1.5molN2ŗĶ4.5molH2,Ź¹·“Ó¦N2+3H2 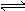 2NH3“ļĘ½ŗā£¬ ²āµĆĘ½ŗā»ģŗĶĘųÖŠN2”¢H2”¢NH3·Ö±šĪŖm”¢n”¢gmol”£Čē¹ūĪĀ¶Č²»±äÖ»øıä³õŹ¼Ź±ø÷ĪļÖŹµÄ¼ÓČėĮ棬±£³ÖĘ½ŗāŹ±m”¢n”¢gÖµ²»±ä£¬ŌņN2”¢H2”¢NH3¼ÓČėĮæÓĆx”¢y”¢z±ķŹ¾£¬Ó¦Āś×ćĢõ¼ž£ŗ
2NH3“ļĘ½ŗā£¬ ²āµĆĘ½ŗā»ģŗĶĘųÖŠN2”¢H2”¢NH3·Ö±šĪŖm”¢n”¢gmol”£Čē¹ūĪĀ¶Č²»±äÖ»øıä³õŹ¼Ź±ø÷ĪļÖŹµÄ¼ÓČėĮ棬±£³ÖĘ½ŗāŹ±m”¢n”¢gÖµ²»±ä£¬ŌņN2”¢H2”¢NH3¼ÓČėĮæÓĆx”¢y”¢z±ķŹ¾£¬Ó¦Āś×ćĢõ¼ž£ŗ
¢ÅČōx=0”¢y=0, Ōņz= mol£»
¢ĘČōx=0.75mol, Ōņy= mol, z= mol£»
¢ĒÓ¦Āś×ćµÄŅ»°ćĢõ¼žŹĒ £» ”£
(ÓĆĮ½øö·½³ĢŹ½±ķŹ¾£¬ĘäÖŠŅ»øöÖ»ŗ¬x”¢z£¬ĮķŅ»øöÖ»ŗ¬y”¢z)

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| 553”«573K |
| 1373K |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģɽ¶«Ź”×Ķ²©Ņ»ÖŠøßČż12ŌĀ½×¶ĪŠŌ¼ģ²ā»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
(¹²12·Ö)
£Ø1£©ŌŚÄ³ČŻ»ż²»±äµÄĆܱÕČŻĘ÷ÖŠ£¬ÓŠæÉÄę·“Ó¦£ŗmA£Øg£©+nB£Øg£© pC£Øg£©+qD£ØS£©”÷H£¼0ČēĶ¼Ä³·“Ó¦¹ż³ĢÖŠø÷ĪļÖŹĪļÖŹµÄĮæn£Ømol£©Ėꏱ¼ätµÄ±ä»ÆĒśĻßĶ¼”£
pC£Øg£©+qD£ØS£©”÷H£¼0ČēĶ¼Ä³·“Ó¦¹ż³ĢÖŠø÷ĪļÖŹĪļÖŹµÄĮæn£Ømol£©Ėꏱ¼ätµÄ±ä»ÆĒśĻßĶ¼”£
¢ŁøĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖ£Øø÷ĪļÖŹÅØ¶ČµÄÖøŹżÓĆŹżÖµ±ķŹ¾£©£ŗ ”£
¢ŚČō³äČėA£¬KÖµ £ØĢīŅ»¶ØŌö“ó”¢Ņ»¶Ø¼õŠ””¢»ņæÉÄÜŌö“óŅ²æÉÄܼõŠ””¢²»±ä£©£»Õż·“Ó¦ĖŁĀŹ____£ØĢīŌö“󔢼õŠ””¢²»±ä£©”£
¢ŪČōĢå»żĪŖ10ÉżµÄĆܱÕČŻĘ÷ÖŠ£¬500”ę”¢ÓŠ“߻ƼĮ“ęŌŚµÄĢõ¼žĻĀ£¬øł¾ŻĶ¼Ź¾»Ų“šĻĀĮŠĪŹĢā£ŗŌŚ0”«15minÄŚµÄĘ½¾ł·“Ó¦ĖŁĀŹ£ŗv£ØB£©=
£Ø2£©¶ŌÓŚÄ³æÉÄę·“Ó¦£ŗA£Øg£©+B£Øg£© 2C£Øg£© ”÷H£¼0”£ČōøĆ·“Ó¦µÄÕż·“Ó¦ĖŁĀŹÓėŹ±¼äµÄ¹ŲĻµČēĶ¼ĖłŹ¾”£ŌŚĘäĖüĢõ¼ž²»±äµÄĒéæöĻĀ£¬ĒėĢīæÕ£ŗ
2C£Øg£© ”÷H£¼0”£ČōøĆ·“Ó¦µÄÕż·“Ó¦ĖŁĀŹÓėŹ±¼äµÄ¹ŲĻµČēĶ¼ĖłŹ¾”£ŌŚĘäĖüĢõ¼ž²»±äµÄĒéæöĻĀ£¬ĒėĢīæÕ£ŗ
¢ŁŠ“³öt2Ź±øıäµÄĢõ¼žæÉÄÜŹĒ£ŗ £ØÓĆĪÄ×Ö±ķ“ļ£©£»
¢Śt4Ź±øĽ»µÄĢõ¼žæÉÄÜŹĒ £ØÓƱąŗűķŹ¾£¬¶ąŃ”æŪ·Ö£©
A£®Ōö“óŃ¹Ēæ B£®¼õŠ”Ń¹Ēæ
C£®Ź¹ÓĆ“ß»Æ¼Į D£®ÉżøßĪĀ¶Č
E£®Ōö“óAµÄÅضČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğÉĻŗ£ŹŠĘÕĶÓĒųøßČżÉĻѧʌ֏Įæµ÷ŃŠ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
¹č¼°Ęä»ÆŗĻĪļ¹ć·ŗÓ¦ÓĆÓŚĢ«ŃōÄܵĥūÓĆ”¢¹āµ¼ĻĖĪ¬¼°¹čĻš½ŗµÄÖʱøµČ”£
“æ¾»µÄ¹čŹĒ“Ó×ŌČ»½ēÖŠµÄŹÆÓ¢æóŹÆ£ØÖ÷ŅŖ³É·ÖĪŖSiO2£©ÖŠĢįČ”µÄ”£øßĪĀĻĀÖĘČ”“æ¹čÓŠČēĻĀ·“Ó¦£Ø·½·Ø1£©£ŗ
¢ŁSiO2(s)+2C(s) Si(s)+2CO(g)
Si(s)+2CO(g)
¢ŚSi(s)+2Cl2(g) SiCl4(g)
SiCl4(g)
¢ŪSiCl4(g)+2H2(g) ”śSi(s)+4HCl(g)
Ķź³ÉĻĀĮŠĢīæÕ£ŗ
£Ø1£©¹čŌ×ÓŗĖĶāÓŠ ÖÖ²»Ķ¬Äܼ¶µÄµē×Ó£¬×īĶā²ćµÄpµē×ÓÓŠ ÖÖ×ŌŠż·½Ļņ”£
£Ø2£©¹čÓėĢ¼Ķ¬Ö÷×壬µ„ÖŹµÄ»¹ŌŠŌ£ŗĢ¼ ¹č£ØĢīŠ“”°Ķ¬ÓŚ”±”¢”°ĒæÓŚ”±»ņ”°ČõÓŚ”±£©”£·“Ó¦¢ŁÖ®ĖłŅŌÄܽųŠŠµÄŌŅņŹĒ ”£
£Ø3£©·“Ó¦¢ŚÉś³ÉµÄ»ÆŗĻĪļµÄµē×ÓŹ½ĪŖ £»øĆ·Ö×ÓĪŖ ·Ö×Ó£ØĢīŠ“”°¼«ŠŌ”±»ņ”°·Ē¼«ŠŌ”±£©”£
£Ø4£©Ä³ĪĀ¶ČĻĀ£¬·“Ó¦¢ŚŌŚČŻ»żĪŖVÉżµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠ£¬“ļµ½Ę½ŗāŹ±Cl2µÄÅضČĪŖa mol/L”£Č»ŗóŃøĖŁĖõŠ”ČŻĘ÷ČŻ»żµ½0.5VÉż£¬tĆėŗóÖŲŠĀ“ļµ½Ę½ŗā£¬Cl2µÄÅضČĪŖb mol/L”£Ōņ£ŗa b£ØĢīŠ“”°“óÓŚ”±”¢”°µČÓŚ”±»ņ”°Š”ÓŚ”±£©”£
£Ø5£©ŌŚtĆėÄŚ£¬·“Ó¦¢ŚÖŠv(SiCl4)= £ØÓĆŗ¬a”¢bµÄ“śŹżŹ½±ķŹ¾£©”£
£Ø6£©¹¤ŅµÉĻ»¹æÉŅŌĶعżČēĻĀ·“Ó¦ÖĘČ”“æ¹č£Ø·½·Ø2£©£ŗ
¢ÜSi(“Ö) £«3HCl(g) 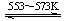 SiHCl3(l)£«H2(g) + Q£ØQ£¾0£©
SiHCl3(l)£«H2(g) + Q£ØQ£¾0£©
¢ŻSiHCl3(g)£«H2(g) Si(“æ)£«3HCl(g)
Si(“æ)£«3HCl(g)
Ģįøß·“Ó¦¢ŻÖŠSi(“æ)µÄ²śĀŹ£¬æɲÉČ”µÄ“ėŹ©ÓŠ£ŗ £ØŃ”Ģī2Ģõ£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com