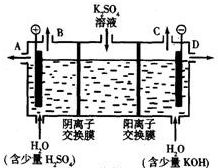
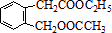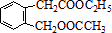ͼ��A��B��C��D��EΪ���ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�������֪��
��C+G��B+H���ų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�G�����Ǵ��������Ҫ�ɷ֣�
��I��һ�ֳ������������壬����E������Ӧ��2E+I��2F+D��F��EԪ�ص���������Ϊ60%��
�ش��������⣮
��1����Ӧ�ٵĻ�ѧ����ʽ��
��
��2��������I�������ڵĻ�ѧ����
��������ӡ����ԡ��Ǽ��ԡ�����
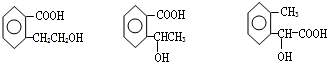
��3����ȡ11.9g B��C��E�Ļ����ù�����NaOH��Һ�ܽ���ˡ�����ʣ���������Ϊ9.2g����ȡ��������B��C��E�Ļ������������ϡ������ȫ�ܽ⣬���ռ������������6.72L����ʣ��Ļ��Һ�м��������NaOH��Һ�����ó���������Ϊ
��
A��27.2g B��7.8g C��2.7g D��19.4g
��4��C�������NaOH��Һ��Ӧ�����ӷ���ʽ�ǣ�
��
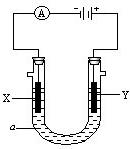
��5����G���ڹ�����ϡ�����У����������е�Fe
3+�ķ�����
���������ѡ����ѡ����ȷѡ���ͬ�������������е�Fe
2+�ķ�����
��
A���μ�KSCN��Һ����Һ��Ѫ��ɫ
B�������ۣ���Һ��dz��ɫ
C����������KMnO
4��Һ��Ѹ����ɫ
D���μ�NaOH��Һ���а�ɫ������Ѹ�ٱ�ɻ���ɫ���ת��Ϊ���ɫ��




 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�

 +2NaOH
+2NaOH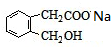 +CH3COONa+CH3CH2OH
+CH3COONa+CH3CH2OH +2NaOH
+2NaOH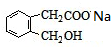 +CH3COONa+CH3CH2OH
+CH3COONa+CH3CH2OH

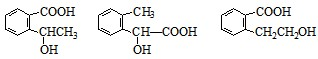 ������һ��
������һ��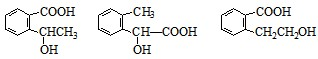 ������һ��
������һ�� ����CH2=CH2+H2O
����CH2=CH2+H2O ����CH2=CH2+H2O
����CH2=CH2+H2O ������ͼ�ش����⣺
������ͼ�ش����⣺

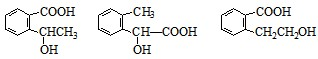 ������һ��
������һ��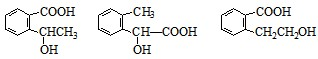 ������һ��
������һ��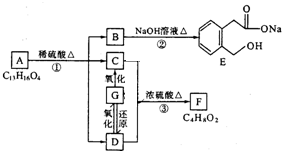 ͼ��A��B��C��D��E��F��G��Ϊ�л������B������ֻ��һ����״�ṹ������ͼʾ�ش����⣺
ͼ��A��B��C��D��E��F��G��Ϊ�л������B������ֻ��һ����״�ṹ������ͼʾ�ش����⣺