
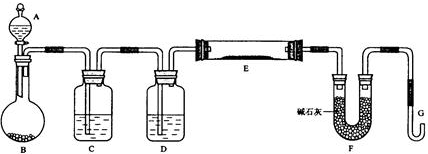
���� ����H2��ԭ��ɫ��CuO��ĩ����ʵ��װ�ÿ�֪��A��Ϊ���ᣬB��ΪZn��C��Ϊˮ�����ջӷ���HCl��D��ΪŨ�������������E�з���H2+CuO$\frac{\underline{\;\;��\;\;}}{\;}$Cu+H2O��F�м�ʯ������ˮ�����H2�������ȿɵ��±�ը�����
��� �⣺����H2��ԭ��ɫ��CuO��ĩ����ʵ��װ�ÿ�֪��A��Ϊ���ᣬB��ΪZn��C��Ϊˮ�����ջӷ���HCl��D��ΪŨ�������������E�з���H2+CuO$\frac{\underline{\;\;��\;\;}}{\;}$Cu+H2O��F�м�ʯ������ˮ��
��1��������������֪��B��C��D�е��Լ��ֱ�ΪZn��ˮ��Ũ���ᣬ�ʴ�Ϊ��Zn��ˮ��Ũ���
��2���÷�ӦΪ������Ʊ�������ʵ�飬�����Ӻ�װ�ú�Ӧ���ȼ��װ�õ������ԣ�����Ϊ��G�����ܷ���ʢˮ��ˮ���У�����B������ˮ����������ð����ֹͣ���Ⱥ�ˮ��G�е��������γ�һ��ˮ�����ʴ�Ϊ�����װ�õ������ԣ���G�����ܷ���ʢˮ��ˮ���У�����B������ˮ����������ð����ֹͣ���Ⱥ�ˮ��G�е��������γ�һ��ˮ����
��3��H2�������ȿɵ��±�ը�����ȴ�Aƿ��εμ�Һ�壬��Ӧһ��ʱ�䣬��G���ռ�������������Ĵ��ȣ��ټ��ȷ�Ӧ��E��
�ʴ�Ϊ����Aƿ��εμ�Һ�壻���������Ĵ��ȣ�
��4��ʵ�������������ʣ�࣬��֤CuO��ȫ����ԭ�����������������������ɵ�ȼ����ֵ���ɫ�Ļ��棨��������ˮ���ռ��ȣ����ʴ�Ϊ����������G�ܳ��ڴ���ȼ����������ˮ���ռ��ȣ���
���� ���⿼���Ʊ�ʵ�鼰����ʵ�鷽������ƣ�Ϊ��Ƶ���㣬���ճ���������Ʊ�ԭ������������ʼ�ʵ��װ�õ�����Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�Ѷ��еȣ�


 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʪ��pH��ֽ��ϡ����Һ��pH���ⶨֵƫ�� | |
| B�� | ������ƿ������Һ������ʱ���ӿ̶��ߣ�������ҺŨ��ƫС | |
| C�� | �ñ���Һ�ζ�δ֪��Һʱ������ʽ�ζ���δ��ϴ����ⶨ���ƫ�� | |
| D�� | �ⶨ�кͷ�Ӧ�ķ�Ӧ��ʱ��������������У�������ƫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʯ��ˮ��ϡ���ᷴӦ��Ca��OH��2+2H+�TCa2++2H20 | |
| B�� | ̼������Һ���������ᷴӦ��CO32-+2H+�TH2O+CO2�� | |
| C�� | ϡ����������������Һ��Ӧ��H++OH-+Ba2++SO42-�TH2O+BaSO4�� | |
| D�� | ������ͭ��ϡ���ᷴӦ��Cu��OH��2+2H+�TCu2++2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ԫ�ص������������ԭ�������ĵ������������Ա仯 | |
| B�� | ����ԭ�������������Ų���ͬ��һ����ͬһ��Ԫ�� | |
| C�� | ԭ�ӵĴ�����������һ����8�� | |
| D�� | һ��ԭ�ӵ�ԭ�Ӻ˶��������Ӻ����ӹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
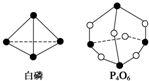 ��ѧ���ļ������γɣ���Ͽ���1mol��ѧ��ʱ�ͷţ������գ�����������֪����P4O6�ķ��ӽṹ��ͼ��ʾ�����ṩ���»�ѧ���ļ��ܣ�kJ•mol-1����P-P��198��P-O��360��O�TO��498������1mol P4O6����ӦP4�����ף�+3O2�TP4O6�е������仯Ϊ��������
��ѧ���ļ������γɣ���Ͽ���1mol��ѧ��ʱ�ͷţ������գ�����������֪����P4O6�ķ��ӽṹ��ͼ��ʾ�����ṩ���»�ѧ���ļ��ܣ�kJ•mol-1����P-P��198��P-O��360��O�TO��498������1mol P4O6����ӦP4�����ף�+3O2�TP4O6�е������仯Ϊ��������| A�� | ����1 638 kJ���� | B�� | �ų�1 638 kJ���� | ||
| C�� | ����126 kJ���� | D�� | �ų�126 kJ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
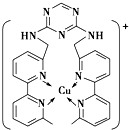 ͭ��̼���������ȵ���������ʵ���ҪԪ�أ�
ͭ��̼���������ȵ���������ʵ���ҪԪ�أ��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com