



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��100�G��
��100�G��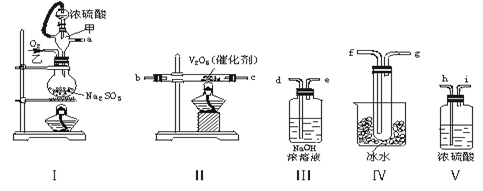
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ϩ����Ȳ | B�����顢�� | C����ϩ���� | D�������屽 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
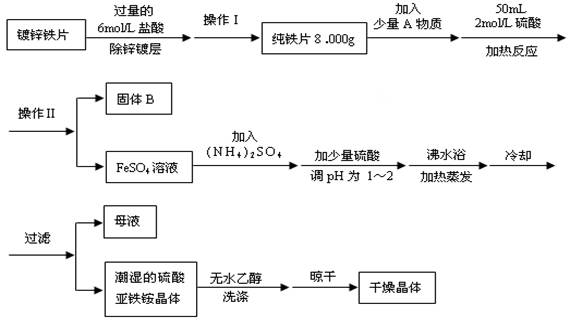
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
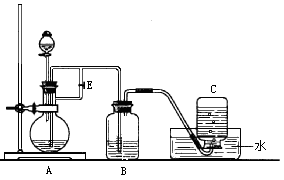
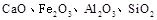 ��ɣ�����ʯ��ʯ��ȡ�Ȼ��ƺ���������ʵ�鲽�����£�
��ɣ�����ʯ��ʯ��ȡ�Ȼ��ƺ���������ʵ�鲽�����£�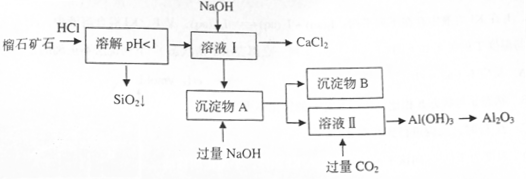
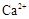 �⣬�����еĽ��������� ��
�⣬�����еĽ��������� ��
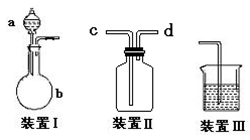
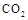 ���岢ͨ����ҺII�У����û�г������������ܵ�ԭ�� ��Ϊ���ܲ���������ͬѧ��ͼIװ�ý����˸Ľ����Ľ��ķ���Ϊ ��
���岢ͨ����ҺII�У����û�г������������ܵ�ԭ�� ��Ϊ���ܲ���������ͬѧ��ͼIװ�ý����˸Ľ����Ľ��ķ���Ϊ ��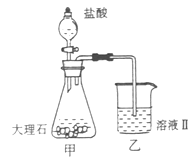
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NH4Cl��H2SO4��Ũ����ϼ��ȣ����ɵ������ü�ʯ�Ҹ��� |
| B��N2 + H2�� NH3�����ռ���� |
| C���Ȼ�狀��������ƹ����ϼ��ȣ������ü�ʯ�Ҹ��� |
| D����Ũ��ˮ�м�����ʯ�ң�������P2O5���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
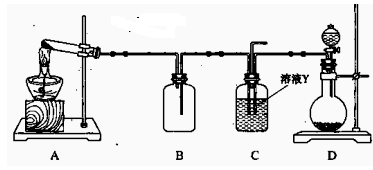
| A��MnO2��Ũ���ᷴӦ�Ʊ�Cl2 | B��Cu��Ũ���ᷴӦ����SO2 |
| C����H2O2�ֽ���O2 | D���Ҵ������ᷴӦ�Ʊ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ���������ʵ�顣��ʵ��װ������ͼ��ʾ��
���������ʵ�顣��ʵ��װ������ͼ��ʾ��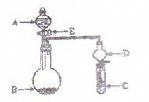
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com