
| A��25��ʱ����Mg��OH��2������Һ�м���������NH4Cl���壬c��Mg2+������ |
| B��һ���¶��£�IL 1mol��L�İ�ˮ��2L 0��5mol��L�İ�ˮ�У�n��NH4+��ǰ�߶� |
| C����ͬ�������ͬ���ʵ���Ũ�ȵ����ᡢ���ᣬϡ����ͬ��������Һ��pH������<���� |
| D��0��2mol��L��һԪ��HX��0��1mol��L��KOH��Һ��������������Һ�У�һ���У�a��H+��+c��K+��=c��OH-��+c��X-�� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| A������Һ�����Ӽ����㣺c(Na+)>c(CH3COO��)>c(OH��)>c(H+)������Һ�����ʿ���ΪCH3COONa��NaOH |
| B������Һ�����Ӽ�����c(CH3COO��)>c(Na+)>c(H+)>c (OH��)������Һ������һ��ֻ��CH3COONa |
| C������Һ��c(Na+)=c(CH3COO��)�������Һһ�������� |
| D������Һ��c(CH3COOH)>c(Na+)������Һһ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na+��H+��Cl����NO3�� | B��K+��Ba2+��OH����I�� |
| C��Na+��Mg2+��Cl����SO42�� | D��Cu2+��S2����Br����ClO�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���μӷ�̪��Һ�Ժ�ɫ����Һ��Fe3+��NH4+��Cl����CO32�� |
| B����������ΪMgCl2����Һ��K+��NO3����SO42����Al3+ |
| C��ˮ���������c(H��)=10��13mol/L����Һ��K+��Na+��HCO3����Br�� |
| D��pH=1����Һ�� Fe2+��Na+��I����NO3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������Ba2������Һ�У�HCO3����Fe3����Ag����SO42����SCN�� |
| B���������ۺ������������Һ�У�NH4����Na����NO3����Cl����S2�� |
| C���μ���ɫ��̪��Һ������ɫ����Һ�У�Na����CO32����K����Cl����AlO2�� |
| D����ˮ�����c(OH��)��10��14mol��L��1����Һ�У�CH3COO����C6H5O����Na����K�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��C(Na+)>C(HCO3-)>C(CO32-)>C(H+)>C(OH-) |
| B��C(Na+) = C(HCO3-) |
| C��C��Na+��+C(H+) = C(HCO3-)+2C(CO32-)+C(OH-) |
| D��C(Na+) = C(HCO3-)+C(CO32-)+C(H2CO3) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na����NO3����Al3����K�� | B��Na����ClO3����ClO����ClO4�� |
| C��K����Cr2O72����I����Na�� | D��Na����S2O32����SO42����NH4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��pH��0����Һ�У�Fe3+��Mg2+��NO3-��SO42- |
| B�����д���Al3+����Һ�У�K+��NH4+��Na+��CO32- |
| C��ˮ�����������Ũ��Ϊ1��10-11 mol��L-1 ��Һ�У�K+��AlO2-��Cl-��HCO3- |
| D���ں�����Fe3+����Һ�У�NH4+��Na+��Cl-��SCN- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��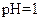 ����Һ�У� ����Һ�У�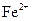 �� ��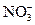 �� ��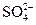 �� �� |
B����ˮ�����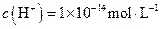 ����Һ�У� ����Һ�У�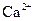 �� �� �� ��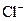 �� ��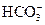 |
C��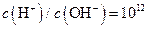 ����Һ�У� ����Һ�У�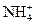 �� ��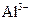 �� ��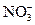 �� ��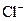 |
D�� ����Һ�У� ����Һ�У� �� ��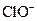 �� ��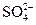 �� �� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com