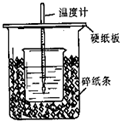
����ͼ��ʾװ�ý����к��Ȳⶨʵ�飬��ش����⣺
��1����С�ձ�֮����������ĭ���ϵ�������
���¡����ȡ�����ʵ������е�����ɢʧ
���¡����ȡ�����ʵ������е�����ɢʧ
����ʵ��װ���Ͽ���ͼ��ȱ�ٵ�һ�ֲ���������
�����������
�����������
��
��2��ʹ�ò�ȫ�������װ�ý���ʵ�飬ȡ50mL0.25mol/LH
2SO
4��Һ��50mL0.55mol/LNaOH��Һ��С�ձ��н����кͷ�Ӧ������ʵ���¶�ƽ������3.4�森��֪�кͺ����ɵ���Һ�ı�����Ϊ4.18J/��g?�棩����Һ���ܶȾ�Ϊ1g/cm
3��ͨ������ɵ��к��ȡ�H=
-56.8KJ/mol
-56.8KJ/mol
��H
2SO
4��NaOH��Ӧ���Ȼ�ѧ����ʽΪ
H
2SO
4��aq��+NaOH��aq��=Na
2SO
4��aq��+H
2O��l������H=-56.8KJ/mol
H
2SO
4��aq��+NaOH��aq��=Na
2SO
4��aq��+H
2O��l������H=-56.8KJ/mol
��
��3��ʵ��������60mL0.25mol?L
-1H
2SO
4��Һ��50mL0.55mol?L
-1NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������
�����
�����
�����ȡ���������ȡ����������к���
���
���
�����ȡ���������ȡ�����������50mL0.50mol?L
-1�������H
2SO
4��Һ��������ʵ�飬��÷�Ӧǰ���¶ȵı仯ֵ��
ƫС
ƫС
���ƫ����ƫС����������Ӱ�족����




 ����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�
����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У� ����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�
����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�