
��һ��ɫ����Һ����ȷ���Ƿ����������ӣ�K����Mg2����Al3����Fe2����Ba2����NO3-��SO42-��Cl����I����HCO3-��ȡ����Һ��������ʵ�飺
| ʵ�鲽�� | ʵ������ |
| ��ȡ��������Һ���Ӽ��μ�����Һ | ��Һ���ɫ |
| ��ȡ��������Һ������ͭƬ��Ũ���ᣬ���� | ����ɫ������������������Ա�ɺ���ɫ |
| ��ȡ��������Һ������BaCl2��Һ | �а�ɫ�������� |
| ��ȡ���е��ϲ���Һ������AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����ϡ���� |
| ��ȡ��������Һ������NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ�����������ܽ� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�й�FeSO4��ת����ϵ��ͼ��ʾ(������������ȥ)��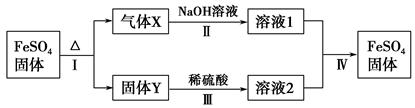
��֪����X�����ֻ�������ɣ���Xͨ��Ʒ����Һ����Һ��ɫ��ͨ��BaCl2��Һ��������ɫ������
��Y�Ǻ���ɫ�Ļ����
(1)����X�ijɷ���(�ѧʽ) ��
(2)��Ӧ��ķ�Ӧ��������(�����) ��
a���ֽⷴӦ b�����ֽⷴӦ
c���û���Ӧ d�����Ϸ�Ӧ
e��������ԭ��Ӧ
(3)��Һ2�н��������ӵļ��鷽���� ��
(4)������Ӧ��õ�16 g����Y������������Xǡ�ñ�0.4 L 1 mol��L��1 NaOH��Һ��ȫ���գ���Ӧ��������FeSO4�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ɼ������ӻ�������ɵĻ����������������е������֣�K����NH4+��Mg2����Ba2����Cl����NO2-��SO42-��CO32-�����û��������ˮ��ó�����Һ����ȡ4��100 mL����Һ�ֱ��������ʵ�飺
| ʵ�� ��� | ʵ������ | ʵ���� |
| A | ��AgNO3��Һ | �а�ɫ�������� |
| B | ������NaOH��Һ������ | �ռ�������1.12 L(������ɱ�״���µ����) |
| C | ������BaCl2��Һ�������ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ�������� | ��һ�γ�����������Ϊ6.27 g���ڶ��γ�����������Ϊ2.33 g |
| D | ������KMnO4������Һ | KMnO4��Һ��ɫ |
| �����ӷ��� | ���ʵ���Ũ��(mol��L��1) |
| | |
| | |
| | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ú����Ҫ����Դ��Ҳ������������Ʒ����Ҫԭ�ϡ�������ѧ֪ʶ������������⣺
(1)ú��ת����������ú������������Һ��������ú��Һ�������ַ�Ϊ________��________��
(2)��úȼ��ǰ���ú������������ú��ij����������ԭ��ΪFeS2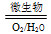 Fe2����SO42��
Fe2����SO42��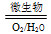 Fe3��������������Ϊ�������������ü����ĵ�һ����Ӧ�����ӷ���ʽΪ_____________________________��
Fe3��������������Ϊ�������������ü����ĵ�һ����Ӧ�����ӷ���ʽΪ_____________________________��
�ڶ�����Ӧ�����ӷ���ʽΪ____________________��
(3)��ҵú����õ��IJ�Ʒ�н�̿��________��
(4)��ҵ����Ҫ���ð��������������ᣬ��ͼ�ǰ��������백������������������ȵĹ�ϵ������ֱ�߱�ʾ��Ӧ������ֵ�����߱�ʾ����ʵ����������������ʴﵽ100%��������r[n(O2)/n(NH3)]��________��ʵ������Ҫ��rֵά����1.7��2.2֮�䣬ԭ����__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������������������γ��������Ҫ���ʡ�ij�������п��ܺ����������ӣ�Na����Ba2����NH4+��Al3����Cl����SO32-��SO42-��NO3-�ȡ�ij�о�С��ȡ�õ�һ���������꣬Ũ�������ó�����Һ�ֳ����ݣ���������ʵ�飺
| ���� | �����Լ� | ʵ������ |
| ��һ����Һ | �μ������ĵ���KI��Һ | ��Һ����ɫ |
| �ڶ�����Һ | �μ��������ữ��BaCl2��Һ | �а�ɫ�������� |
| ��������Һ | �μ�NaOH��Һ�����ȣ������NaOH��Һ���(V)�����ɵij�������������������ʵ���(n)�Ĺ�ϵ����ͼ | 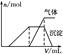 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ȣ�ClO2����Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч����������������һ�ֻ���ɫ�����壬������ˮ��
��.��1��ClO2����KClO3��H2SO4���ڵ���������Na2SO3��Ӧ�Ƶá���÷�Ӧ�����������뻹ԭ��������ʵ���֮����________��
��.ʵ����Ҳ����NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ���Ʊ�ClO2�����������£�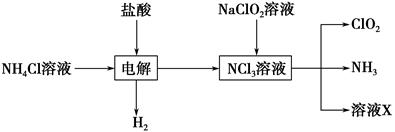
��2��д�����ʱ������Ӧ�Ļ�ѧ����ʽ��________________________________��
��3����ȥClO2�е�NH3��ѡ�õ��Լ���________��������ţ�
| A������ʳ��ˮ | B����ʯ�� |
| C��Ũ���� | D��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ֺ��ؽ��У�����ˮ��ȫռ�м�Ϊ��Ҫ�ĵ�λ��ij�о�С����ȡ��������Ⱦ��ˮԴ���з�����������������ʵ����Ϣ������һ������Ⱦ��ˮԴ����A��B�������ʣ�һ������C��D�������ʣ�һ������E���ʣ�A��B��C��D��E���ֳ��������ﶼ�����±��е������γɵģ�
| ������ | K����Na����Cu2����Al3�� |
| ������ | SO42����HCO3����NO3����OH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ҵ��ˮ�����±��е�ijЩ���ӣ��Ҹ������ӵ����ʵ���Ũ����ȣ���Ϊ0.1 mol/L(����ֵ����ˮ�ĵ��뼰���ӵ�ˮ��)��
| ������ | K����Ag����Mg2����Cu2����Al3����NH4+ |
| ������ | Cl����CO32����NO3����SO42����SiO32����I�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ҫ��ش����и���
�������� ���� ��NH3������������Һ ��Һ̬�Ȼ��� ��AgCl����ޱ����� �����ǣ���ջش�����ţ���
���������У�1�����ڵ���ʵ��� ����2�����ڷǵ���ʵ��� ��
��3������ǿ����ʵ��� ����4���ܵ������ ��
����д�����з�Ӧ�Ļ�ѧ����ʽ��
����NaHCO3���ɵĻ��Ϸ�Ӧ
�� ��MgCl2�μӵķֽⷴӦ
�� ��Fe2O3�μӵ��û���Ӧ
�� ��HNO3���ɵĸ��ֽⷴӦ
(��).ͬ��ͬѹ�����£�ͬ�����CH4��SO2������֮���� ��ͬ������CH4��SO2�����֮���� ������������ԭ�Ӹ���������ȣ���CH4��SO2������֮���� ��
����������Ƭ��ʯī��������a��b���ַ�ʽ����ʢ��ϡ��������Һ�ͷ�̪��Һ�����Һ�IJ��������У�����һ��ʱ������ȹ۲쵽��Һ���������� ������ţ���
| A����͢� | B����͢����� | C����͢� | D����͢����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com