
��ţ��������ϲ����һ��Ӫ�����ϣ���ţ������������ɲ���������л��ᣬʹ���Ƚ��ͣ���Ч�����Ƴ����ڲ����ķ�ֳ����ţ���е����������ʳ�����ٽ�θҺ���ڣ���ǿ��θ���������ܣ���������г��ٺͱ������ã���ҵ�����������ϩ���ϳɣ��������£�
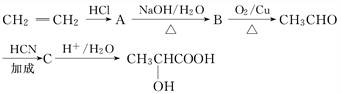
(1)���������Ĺ����ŵ�������_________________________________________��
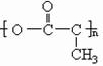
(2)���������ڲ�ͬ�����¿��γɲ�ͬ��������������Է���������С����Ľṹ��ʽ����Ϊ��__________________��__________________��__________________.
(3)д������ת���Ļ�ѧ����ʽ��
A������________________________________________________________��
CH3CHO������______________________________________________________��
C������____________________________________________________________��
A��B�ķ�Ӧ����Ϊ___________________________________________________��
(1)�ǻ����Ȼ� ��2��

(3)CH2===CH2��HCl CH3��CH2Cl
2CH3CH2OH��O2
CH3��CH2Cl
2CH3CH2OH��O2 2CH3CHO��2H2O
2CH3CHO��2H2O
 ��ȡ����Ӧ(��ˮ�ⷴӦ)
��ȡ����Ӧ(��ˮ�ⷴӦ)
��������
�����������������к��Ȼ��ʹ��ǻ������γɻ����������������ȣ��ݴ˿���д���йط�Ӧ�Ļ�ѧ����ʽ��
���㣺�����л�������š��ṹ��ʽ���л���Ӧ���͵��ж��Լ�����ʽ����д
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����������У�������ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ���������������ͷ�ɢ˼ά����������Ĺؼ�����ȷ���ֹ����Žṹ�����ʣ�Ȼ��������������⡢����������ɣ�����������ѧ������ѧ������֪ʶ��Ǩ��������


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
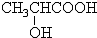 �ܣ���������г��ٺͱ������ã�����ĽṹΪ��
�ܣ���������г��ٺͱ������ã�����ĽṹΪ��| HCl |
| NaOH/H2O |
| �� |
| O2(Cu) |
| �� |
| HCN |
| �ӳ� |
| H+/H2O |
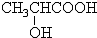
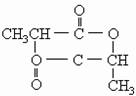

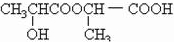
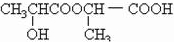



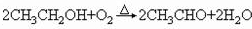
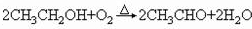
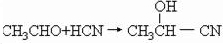
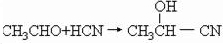
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ţ��������ϲ����һ��Ӫ�����ϡ���ţ������������ɲ���������л��ᣬʹ���Ƚ��ͣ���Ч�����Ƴ����ڲ����ķ�ֳ����ţ���е����������ʳ�����ٽ�θҺ���ڣ���ǿ��θ���������ܣ���������г��ٺͱ������ã�����ĽṹΪ��
��ҵ����������ϩ���ϳɣ��������£�
��1�����������Ĺ����ŵ������� ��
��2�����������ڲ�ͬ�����¿��γɲ�ͬ������������ʽ����С����Ľṹ��ʽ����Ϊ��
�� �� ��
��3��д������ת���Ļ�ѧ����ʽ��A������ ��CH3CHO������ ��C������ ��A��B�ķ�Ӧ����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭��һ�и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��ţ��������ϲ����һ��Ӫ�����ϡ���ţ������������ɲ���������л��ᣬʹ���Ƚ��ͣ���Ч�����Ƴ����ڲ����ķ�ֳ����ţ���е����������ʳ�����ٽ�θҺ���ڣ���ǿ��θ���������ܣ���������г��ٺͱ������ã�����ĽṹΪ��
��ҵ����������ϩ���ϳɣ��������£�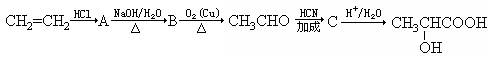
��1�����������Ĺ����ŵ������� ��
��2�����������ڲ�ͬ�����¿��γɲ�ͬ������������ʽ����С����Ľṹ��ʽ����Ϊ��
�� �� ��
��3��д������ת���Ļ�ѧ����ʽ��A������ ��CH3CHO������ ��C������ ��A��B�ķ�Ӧ����Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com