
·ÖĪö £Ø1£©A£®ŅĄ¾ŻÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄŅ»°ć²½ÖčŃ”ŌńŠčŅŖµÄŅĒĘ÷£»
B£®ŅĄ¾Żm=CVM¼ĘĖćŠčŅŖČÜÖŹµÄÖŹĮ棻
£Ø2£©·ÖĪö²Ł×÷¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»żµÄÓ°Ļģ£¬ŅĄ¾ŻC=$\frac{n}{V}$½ųŠŠĪó²ī·ÖĪö£®
½ā“š ½ā£ŗ£Ø1£©A£®ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄŅ»°ć²½Öč£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ£¬ÓƵ½µÄŅĒĘ÷£ŗĶŠÅĢĢģĘ½”¢Ņ©³×”¢ÉÕ±”¢²£Į§±”¢ČŻĮæĘ攢½ŗĶ·µĪ¹Ü£»ÅäÖĘ490mL 0.10mol•L-1µÄNaOHČÜŅŗ£¬Ó¦Ń”Ōń500mLČŻĮæĘ棬ĖłŅŌӦєÓĆ²£Į§ŅĒĘ÷ÓŠ£ŗ²£Į§°ō”¢½ŗĶ·µĪ¹Ü”¢500mlµÄČŻĮæĘ攢ÉÕ±£»
¹Ź“š°øĪŖ£ŗA£®²£Į§°ō”¢½ŗĶ·µĪ¹Ü”¢500mlµÄČŻĮæĘ攢ÉÕ±£»
B£®ÅäÖĘ490mL 0.10mol•L-1µÄNaOHČÜŅŗ£¬Ó¦Ń”Ōń500mLČŻĮæĘ棬ŠčŅŖČÜÖŹµÄÖŹĮæm=0.10mol/L”Į0.5L”Į40g/mol=2.0g£»
¹Ź“š°øĪŖ£ŗ2.0£»
£Ø2£©A£®¶ØČŻŹ±ø©ŹÓæĢ¶ČĻߣ¬µ¼ÖĀČÜŅŗĢå»żĘ«Š”£¬ČÜŅŗÅضČĘ«øߣ¬¹ŹA²»Ń”£»
B£®½«ÉÕ±ÖŠČܽāŗóµÄČÜŅŗĮ¢æĢ×¢ČėČŻĮæĘæ£¬Č»ŗóŌŁĢķ¼ÓÕōĮóĖ®ÖĮæĢ¶ČĻߣ¬ĄäČ“ŗóČÜŅŗĢå»żĘ«Š”£¬ČÜŅŗÅضČĘ«øߣ¬¹ŹB²»Ń”£»
C£®Ņ”ŌȶØČŻŗó£¬ÓĆ½ŗĶ·µĪ¹ÜĻņČŻĮæĘæÖŠµĪ¼ÓÕōĮóĖ®ÖĮæĢ¶ČĻߣ¬µ¼ÖĀČÜŅŗĢå»żĘ«“ó£¬ČÜŅŗÅضČĘ«µĶ£¬¹ŹCŃ”£»
D£®ÅäÖĘČÜŅŗĒ°ÓĆÕōĮóĖ®ČóĻ“ČŻĮæĘ棬¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»ż¶¼²»»į²śÉśÓ°Ļģ£¬ČÜŅŗÅØ¶Č²»±ä£¬¹ŹD²»Ń”£»
E£®¶ØČŻŹ±³¬³öæĢ¶ČĻߣ¬ÓĆ½ŗĶ·µĪ¹Ü½«ČÜŅŗĪü³öµ½æĢ¶ČĻߣ¬µ¼ÖĀ²æ·ÖČÜÖŹĖšŗÄ£¬ČÜÖŹµÄĪļÖŹµÄĮæĘ«Š”£¬ČÜŅŗÅضČĘ«µĶ£¬¹ŹEŃ”£»
F£®ŅĘŅŗ¹ż³ĢÖŠ²»Š”ŠÄ½«ČÜŅŗ½¦³ö£¬µ¼ÖĀ²æ·ÖČÜÖŹĖšŗÄ£¬ČÜÖŹµÄĪļÖŹµÄĮæĘ«Š”£¬ČÜŅŗÅضČĘ«µĶ£¬¹ŹFŃ”£»
¹ŹŃ”£ŗC EF£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ£¬Ć÷Č·ÅäÖĘŌĄķ¼°²Ł×÷²½ÖčŹĒ½āĢā¹Ų¼ü£¬×¢ŅāĪó²ī·ÖĪöµÄ·½·ØŗĶ¼¼ĒÉ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NaHSO3ŗĶNaHCO3µÄ»ģŗĻČÜŅŗÖŠ£ØSŗĶC¾łÓĆR±ķŹ¾£©£ŗc£ØNa+£©+c£ØH+£©=c£ØHRO3-£©+c£ØRO32-£©+c£ØOH-£© | |
| B£® | ³£ĪĀĻĀĪļÖŹµÄĮæÅضČĻąµČµÄ¢Ł£ØNH4£©2CO3”¢¢Ś£ØNH4£©2SO4”¢¢Ū£ØNH4£©2Fe£ØSO4£©2ČżÖÖČÜŅŗÖŠĖ®µÄµēĄė³Ģ¶Č£ŗ¢Ū£¾¢Ł£¾¢Ś | |
| C£® |  ³£ĪĀĻĀ“×ĖįŗĶ“×ĖįÄĘ»ģŗĻČÜŅŗÖŠc£ØCH3COOH£©”¢c£ØCH3COO-£©ÓėpHÖµµÄ¹ŲĻµČēĶ¼ĖłŹ¾£¬µ±pH=4.5ČÜŅŗÖŠ£ŗc£ØCH3COOH£©£¾c£ØCH3COO-£©£¾c£ØH+£©£¾c£ØOH-£© | |
| D£® | ±ł“×ĖįÖŠÖšµĪ¼ÓĖ®£¬ČÜŅŗµÄµ¼µēŠŌ”¢“×ĖįµÄµēĄė³Ģ¶Č”¢pH¾łĻČŌö“óŗó¼õŠ” |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

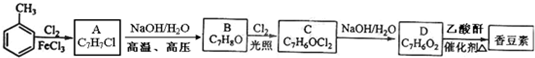
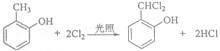 £®
£®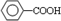 £ØŠ“½į¹¹¼ņŹ½£©£®
£ØŠ“½į¹¹¼ņŹ½£©£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
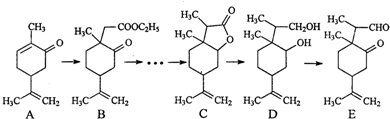
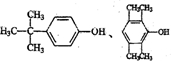 £ØČĪŠ“Ņ»ÖÖ£©£®
£ØČĪŠ“Ņ»ÖÖ£©£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | ²¤ĀÜõ„²»“ęŌŚĖ³·“Ņģ¹¹ĻÖĻó | B£® | ŌĮĻÖŠ×ī¶ą5øöŌ×Ó¹²Ļß | ||
| C£® | ²¤ĀÜõ„ÖŠĖłÓŠµÄŌ×Ó¶¼æÉÄܹ²Ę½Ćę | D£® | ÖŠ¼äĢåŗĶ²¤ĀÜõ„ÖŠ¾łŗ¬ŹÖŠŌĢ¼Ō×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Fe3++3H2OØTFe£ØOH£©3+3H+ | B£® | Br-+H2O?HBr+OH- | ||
| C£® | CO32-+2H2O?H2CO3+2OH- | D£® | NH4++H2O?NH3•H2O+H+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
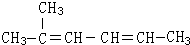 2-¼×»ł-2£¬4-¼ŗ¶žĻ©£®
2-¼×»ł-2£¬4-¼ŗ¶žĻ©£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
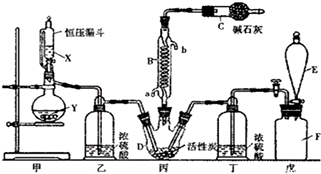
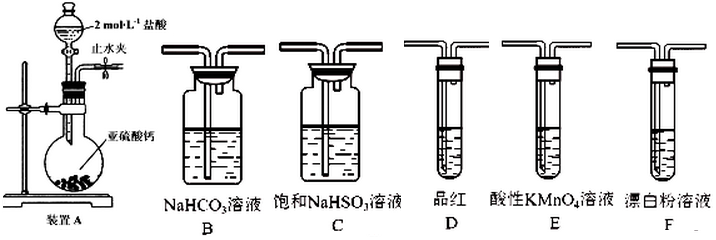
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Mg | B£® | C | C£® | Si | D£® | S |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com