
| Ԫ������ ��� | �� | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶��10-10m�� | 0.74 | 0.89 | 0.82 | 0.77 | 0.99 | 0.75 | 1.17 | 1.43 |
| �����ͻ��ϼ� | +2 | +3 | +4 | +7 | +5 | +4 | +3 | |
| -2 | -4 | -1 | -3 | -4 |

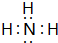 ������������ˮ�����Һ�ʼ��ԣ���ᡱ��������С�����
������������ˮ�����Һ�ʼ��ԣ���ᡱ��������С��������� ��û����ۡ�ֻ����ͼ�-2������֪��ΪO���ۢ�����+3�����ڢ�A�壬ԭ�Ӱ뾶�ۣ��࣬�ʢ�ΪB����ΪAl���ܢ߶������+4����ͼ�-4�����ڢ�A�壬�Ңܵ�ԭ�Ӱ뾶��С���ʢ�ΪC����ΪSi���������+7����ͼ�-1�����ΪCl����ֻ�������+2�����ڢ�A�壬ԭ�Ӱ뾶С��Cl���ʢ�ΪBe���������+5����ͼ�-3�����ڢ�A�壬ԭ�Ӱ뾶С��Cl���ʢ�ΪN���ݴ˽��
��� �⣺��û����ۡ�ֻ����ͼ�-2������֪��ΪO���ۢ�����+3�����ڢ�A�壬ԭ�Ӱ뾶�ۣ��࣬�ʢ�ΪB����ΪAl���ܢ߶������+4����ͼ�-4�����ڢ�A�壬�Ңܵ�ԭ�Ӱ뾶��С���ʢ�ΪC����ΪSi���������+7����ͼ�-1�����ΪCl����ֻ�������+2�����ڢ�A�壬ԭ�Ӱ뾶С��Cl���ʢ�ΪBe���������+5����ͼ�-3�����ڢ�A�壬ԭ�Ӱ뾶С��Cl���ʢ�ΪN��
��1���ڵ�Ԫ�ط�����Be���۵�Ԫ�������ǣ��𣻢�ΪOԪ�أ���Ԫ�����ڱ��е�λ���ǣ��ڶ����ڢ�A�壬�������ΪAl3+�����ӽṹʾ��ͼ�� ��
��
�ʴ�Ϊ��Be���𣻵ڶ����ڢ�A�壻 ��
��
��2���ݺ͢�����������Ӧˮ����ֱ�ΪHClO4��HNO3�������Դ�С��HClO4��HNO3���ʴ�Ϊ��HClO4��HNO3��
��3���͢ߵ��⻯��ֱ�ΪNH3��SiH4���ǽ�����N��Si�����⻯����ȶ��ԣ�NH3��SiH4��NH3���⻯�����ʽΪ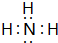 ������������ˮ�����Һ�ʼ��ԣ�
������������ˮ�����Һ�ʼ��ԣ�
�ʴ�Ϊ��NH3��SiH4�� ���
���
��4��������������Ӧˮ����ΪHNO3�������⻯��ΪNH3�����߷�Ӧ�Ļ�ѧ����ʽ��NH3+HNO3=NH4HNO3���ʴ�Ϊ��NH3+HNO3=NH4HNO3��
��5���������������Ӧˮ����ΪAl��OH��3���������м�����ǿ����������ΪNaOH�����߷�Ӧ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��6���ܵ��⻯��ΪCH4����ݵĵ���Cl2�������Ϊ1��2���ڹ��������·�Ӧ�����ɵ��л������У�һ�ȼ��顢���ȼ��顢���ȼ��顢���ȼ���4���л�����г��������������CH3Cl������4����ͬ���Լ����� CCl4��
�ʴ�Ϊ��4��CH3Cl��CCl4��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��Ѷ��еȣ��ؼ��Ǹ��ݻ��ϼ���ԭ�Ӱ뾶�ƶ�Ԫ�أ�ע��Ԫ�������ɵ��������գ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
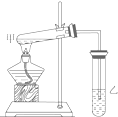 ��ͼ��ijѧ����Ƶ���ȡ����������ʵ��װ��ͼ��ʵ���в�ȡ��������Ҫʵ�������
��ͼ��ijѧ����Ƶ���ȡ����������ʵ��װ��ͼ��ʵ���в�ȡ��������Ҫʵ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 C60��N60Ϊ�·��ֵ���������;�ĵ��ʡ����ӽṹ����ͼ��ʾ���о�������C60��N60���������о��ﵽ8�����ȶ��ṹ��
C60��N60Ϊ�·��ֵ���������;�ĵ��ʡ����ӽṹ����ͼ��ʾ���о�������C60��N60���������о��ﵽ8�����ȶ��ṹ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ����ʽ | ������ | ��Ŀ |
| A | C2H4O2 | ����Na2CO3��Һ��Ӧ | 2 |
| B | C4H8Cl2 | ��������һ���� | 3 |
| C | C4H10O | �������Ʒ�Ӧ | 2 |
| D | C8H10 | ����� | 3 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Ni��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 9.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
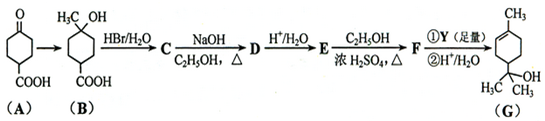
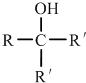
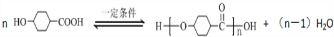
 ���ٺ˴Ź���������2�����շ� ���ܷ���������Ӧ
���ٺ˴Ź���������2�����շ� ���ܷ���������Ӧ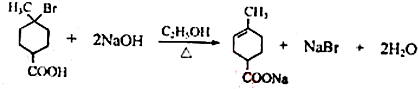 ��
��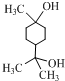 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com