
(��С��15��) A��B��C��D��Ԫ�صĺ˵�����������ӣ����ǵ����ӵĵ��Ӳ�����ͬ��������������Ϊ8��Aԭ�ӵ�L���������K��M�������֮����ȣ�Dԭ�ӵ�K��L�������֮�͵��ڵ���������һ�롣�ش��������⣺
(1)��Ԫ�صķ���������A______��B______��C________��D______��
(2)д��B��DԪ�ص����ӽṹʾ��ͼ������B��______________________�� D��______________________��
(3)�õ���ʽ��ʾA��CԪ���γɵ����ӻ�����Ĺ��̣� ________________________________��
(4)��A��B��C��D���������ˮ�����У�
�Ƚ������������ǿ����____________________________��
�Ƚ����м�ļ���ǿ����____________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(��С��15��)Ϊ�ⶨ������CO2������������ʵ�顣
| ��0.1mol/L�ı������0.01mol/L�ı����� | �� | ��0.1mol/L�ı�����ζ�δ֪Ba(OH)2��Һ10mL��ȥ����19.60 mL | �� | ��Ba(OH)2��Һ���տ����е�CO2 | �� | �� �� | �� | ȡ��Һ20mL����0.01mol/L������ζ���ȥ����34.8mL |
��1��Ϊ���ñ���Һ����ѡȡ�����һ������ ��
��������ƽ ������ƿ �۵ζ��� ����Ͳ ���ձ� ��ͷ�ι� �߲�����
A���٢ڢݢ� B���ڢܢݢޢ� C���ڢ٢ޢ� D���ڢܢݢ�
��2���ζ�����ʱ������ ���۾�ע�� ��
��3��ȡ����Ba(OH)2��Һ10mL����100mL����ƿ�У���ˮϡ�����̶ȣ���ϡ�ͺ����Һ�����ܱ�������������10L�����������ˡ�����˵�ԭ���� ��
��4����ʵ���������������CO2���������Ϊ ��
��5����ʵ���У�����һ�εζ�ʱʹ�õ���ʽ�ζ���δ����������������Һ�����еڶ��εζ�������ʵ������ֵ����ƫ�ߡ�ƫ�ͻ���Ӱ�죩 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(��С��15��) A��B��C��D��Ԫ�صĺ˵�����������ӣ����ǵ����ӵĵ��Ӳ�����ͬ��������������Ϊ8��Aԭ�ӵ�L���������K��M�������֮����ȣ�Dԭ�ӵ�K��L�������֮�͵��ڵ���������һ�롣�ش��������⣺
����(1)��Ԫ�صķ���������A______��B______��C________��D______��
����(2)д��B��DԪ�ص����ӽṹʾ��ͼ��
����B��______________________�� D��______________________��
(3)�õ���ʽ��ʾA��CԪ���γɵ����ӻ�����Ĺ��̣�
________________________________________________________��
(4)��A��B��C��D���������ˮ�����У�
�Ƚ������������ǿ����____________________________��
�Ƚ����м�ļ���ǿ����____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(��С��15��)�ö������ȣ�ClO2���������ƣ�Na2FeO4Ħ������Ϊ166 g��mol��1�������;�ˮ�������ͳ�ľ�ˮ��Cl2�Ե�ˮ���������dz�������ˮ�����¼�����ClO2��Na2FeO4��ˮ���������зֱ𱻻�ԭΪCl����Fe3����
(1)����Ե�λ���������������õ��ĵ���������ʾ����Ч�ʣ���ô��ClO2��Na2FeO4��Cl2��������ɱ����������Ч���ɴ�С��˳���� �� �� ��
(2)������֮�����ܾ�ˮ��������������ǿ�������⣬��һ��ԭ������ǣ�
��

(3)����������һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ��59�棬�е�Ϊ11.0�棬������ˮ��ClO2���Կ����������ᣨHClO2�������ᣨHClO3���Ļ����������ҵ�����Գ�ʪ��KClO3�Ͳ�����60��ʱ��Ӧ�Ƶá�ijѧ������ͼ��ʾ��װ��ģ�ҵ��ȡ���ռ�ClO2������AΪClO2�ķ���װ�ã�BΪClO2������װ�ã�CΪβ������װ�á����ʣ�
��A���ֻ�Ӧ�����¶ȿ��ƣ���ˮԡ���ȣ�װ�ã�B���ֻ�Ӧ����ʲôװ�� ��
��C��Ӧװ���Լ�Ϊ ��C�з�����Ӧ�Ļ�ѧ����ʽΪ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(��С��15��)��֪һ�������±�ϩ��ŨHBr�ܷ������·�Ӧ��
CH3��CH��CH2 + H��Br CH3����CH3
������A��A����Ϊͬ���칹�壬Ԫ�ط�������58.4%������Ԫ�����ݲ�ȫ����ͬ����A��A��������ϵ�з�Ӧ��������̬����B��������̼85.7%��C������������ͼ��C��ϵ��ת����EΪ�������ʣ�����̼�⺬����Ϊ63.6%��
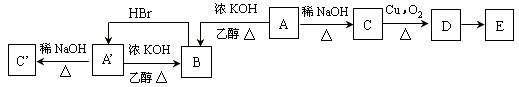
��ش��������⣺
(1)д���ṹ��ʽ��A ��B�� ��
(2)д������ת����ϵ�Ļ�ѧ����ʽ��
��B��A�� �� ��
��C��D�� ��
��D��E�� ��
(3)��������A�к�����Ԫ�ص�ʵ�鷽����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(��С��15��) A��B��C��D����Ԫ��ԭ�Ӻ˵������������С��20�����䵥�ʼ���Ӧ�Ļ������ܷ������·�Ӧ��ϵ��
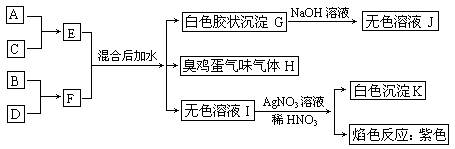
(1)д������Ԫ��Ԫ�ط��ţ�A��____B��____C��____D��____��
(2) F�ĵ���ʽ��_________________��
(3)д��E��F��Ϻ��ˮ�Ļ�ѧ����ʽ��_________________________________��
(4)д��GJ�Ļ�ѧ����ʽ��_________________________________��
(5)д��C������Hˮ��Һ��Ӧ�����ӷ���ʽ��_____________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com