
ЁОЬтФПЁПЮвЙњЛЏЙЄзЈМвКюЕТАёЕФЁАКюЪЯжЦМюЗЈЁБдјЮЊЪРНчжЦМюЙЄвЕзіГіСЫЭЛГіЙБЯзЁЃЫћвдNaClЁЂNH3ЁЂCO2ЕШЮЊдСЯЯШжЦЕУNaHCO3ЃЌНјЖјЩњВњГіДПМюЁЃгаЙиЗДгІЕФЛЏбЇЗНГЬЪНШчЯТЃК
NH3ЃЋCO2ЃЋH2O=NH4HCO3 NH4HCO3ЃЋNaCl=NaHCO3Ё§ЃЋNH4Cl 2NaHCO3![]() Na2CO3ЃЋCO2ЁќЃЋH2O
Na2CO3ЃЋCO2ЁќЃЋH2O
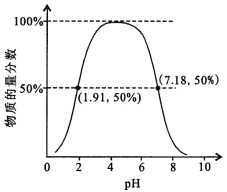
ЃЈ1ЃЉ ЬМЫсЧтяЇгыБЅКЭЪГбЮЫЎЗДгІЃЌФмЮіГіЬМЫсЧтФЦОЇЬхЕФдвђЪЧ________ЃЈЬюзжФИЃЉЁЃ
aЬМЫсЧтФЦФбШмгкЫЎ
b ЬМЫсЧтФЦЪмШШвзЗжНт
c ЬМЫсЧтФЦЕФШмНтЖШЯрЖдНЯаЁЃЌЫљвддкШмвКжаЪзЯШНсОЇЮіГі
ЃЈ2ЃЉ ФГЬНОПЛюЖЏаЁзщИљОнЩЯЪіжЦМюдРэЃЌНјааЬМЫсЧтФЦЕФжЦБИЪЕбщЃЌЭЌбЇУЧАДИїздЩшМЦЗНАИЪЕбщЁЃ
ЂйвЛЮЛЭЌбЇНЋЖўбѕЛЏЬМЦјЬхЭЈШыКЌАБЕФБЅКЭЪГбЮЫЎжажЦБИЬМЫсЧтФЦЃЌЪЕбщзАжУШчЯТЭМЫљЪОЃЈЭМжаМаГжЁЂЙЬЖЈгУЕФвЧЦїЮДЛГіЃЉЁЃ
ЃЈЂёЃЉввзАжУжаЕФЪдМСЪЧ________________ЁЃ
ЃЈЂђЃЉЪЕбщНсЪјКѓЃЌЗжРыГіNaHCO3ОЇЬхЕФВйзїЪЧ________ЃЈЬюУћГЦЃЉЁЃ
ЂкСэвЛЮЛЭЌбЇгУЭМжаЮьзАжУЃЈЦфЫћзАжУЮДЛГіЃЉНјааЪЕбщЁЃ
ЃЈЂёЃЉЪЕбщЪБЃЌаыЯШДг________ЙмЭЈШы________ЦјЬхЃЌдйДг________ЙмЭЈШы________ЦјЬхЁЃ
ЃЈЂђЃЉгаЭЌбЇНЈвщдкЮьзАжУЕФbЙмЯТЖЫСЌНгМКзАжУЃЌРэгЩЪЧ__________ЁЃ
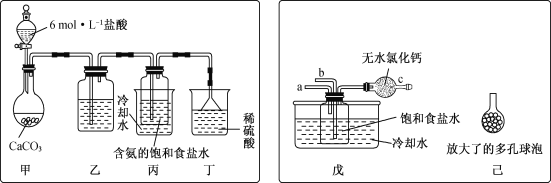
ЁОД№АИЁПc БЅКЭЬМЫсЧтФЦШмвК Й§ТЫ a NH3 b CO2 діДѓЦјЬхгыШмвКЕФНгДЅУцЛ§ЃЌЬсИпCO2ЮќЪеТЪ
ЁОНтЮіЁП
ЙЄвЕЩЯКюЪЯжЦМюЗЈЪЧдкБЅКЭЪГбЮЫЎжаЭЈШыАБЦјКЭЖўбѕЛЏЬМЃЌгЩгкАБЦјдкЫЎжаЕФШмНтЖШДѓЃЌЫљвдЯШЭЈШыАБЦјЃЌЭЈШызуСПЕФАБЦјКѓдйЭЈШыЖўбѕЛЏЬМЃЌЩњГЩСЫЬМЫсЧтФЦЃЌгЩгкЬМЫсЧтФЦЕФШмНтЖШНЯаЁЃЌЫљвдШмвКжагаЬМЫсЧтФЦОЇЬхЮіГіЃЌНЋЬМЫсЧтФЦОЇЬхМгШШКѓЕУДПМюЬМЫсФЦЁЃ
ЃЈ1ЃЉaЁЂЬМЫсЧтФЦвзШмгкЫЎЃЌЙЪДэЮѓЃЛ
BЁЂЬМЫсЧтФЦЪмШШвзЗжНтЃЌгыЦфдкШмвКжаЪзЯШНсОЇЮіГіЮоЙиЃЌЙЪДэЮѓЃЛ
CЁЂЬМЫсЧтФЦЕФШмНтЖШЯрЖдгкТШЛЏяЇРДЫЕЬМЫсЧтФЦЕФШмНтЖШИќаЁвЛаЉЃЌЫљвддкШмвКжаЪзЯШНсОЇЮіГіЃЌЙЪе§ШЗЃЛ
Cе§ШЗЃЌЙЪД№АИЮЊЃКcЃЛ
ЃЈ2ЃЉЂйЃЈIЃЉРћгУбЮЫсжЦШЁЖўбѕЛЏЬМЪБЃЌвђбЮЫсвзЛгЗЂЃЌЫљвдЃЌЖўбѕЛЏЬМжаГЃЛсКЌгаТШЛЏЧтЦјЬхЃЌЬМЫсЧтФЦФмгыбЮЫсЗДгІВЛгыЖўбѕЛЏЬМЗДгІЃЌЫљвдЭЈЙ§ЬМЫсЧтФЦЕФШмвКЪЧПЩвдГ§ЕєЖўбѕЛЏЬМЦјЬхжаЕФТШЛЏЧтЦјЬхЃЌЙЪД№АИЮЊЃКБЅКЭЬМЫсЧтФЦШмвКЃЛ
ЃЈIIЃЉЗжРыГіNaHCO3ОЇЬхЕФВйзїЪЧЗжРыЙЬЬхгывКЬхЃЌГЃВЩгУЕФЪЕбщВйзїЪЧЙ§ТЫВйзїЃЌЙЪД№АИЮЊЃКЙ§ТЫЃЛ
ЂкЃЈIЃЉжЦШЁЬМЫсЧтФЦЪБЯШвЊЕУЕНКЌАБЕФБЅКЭЪГбЮЫЎЃЌАБЦјМЋвзШмгкЫЎЃЌЖўбѕЛЏЬМФмШмгкЫЎЃЌЫљвдгІЯШЭЈШыАБЦјЃЌЫљвдaЖЫЭЈШыЃЌДгЖјБЃжЄСЫДгbЭЈШыЖўбѕЛЏЬМЪБЃЌЖўбѕЛЏЬМБЛГфЗжЗДгІЃЌЙЪД№АИЮЊЃКaЃЛNH3ЃЛbЃЛCO2ЃЛ
ЃЈIIЃЉзАжУИФЖЏКѓЗДгІЮяЕФЖўбѕЛЏЬМгыШмвКЕФНгДЅУцЛ§БфДѓЃЌЬсИпСЫЖўбѕЛЏЬМЕФЮќЪеТЪЃЌ
ЙЪД№АИЮЊЃКдіДѓЦјЬхгыШмвКНгДЅУцЛ§ЃЌЬсИпCO2ЮќЪеТЪЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯжвдЕэЗлЛђввЯЉЮЊжївЊдСЯЖМПЩвдКЯГЩввЫсввѕЅЃЌЦфКЯГЩТЗЯпШчЭМЫљЪОЁЃ

ЃЈвбжЊЃК2CH3CHO+O2![]() 2CH3COOHЃЉ
2CH3COOHЃЉ
ЃЈ1ЃЉAжаКЌгаЕФЙйФмЭХУћГЦЪЧ______________ЃЛЦфжаЂлЕФЗДгІРраЭЪЧ______________ЃЛ
ЂоЕФЗДгІРраЭЪЧ______________ЃЛ
ЃЈ2ЃЉаДввЯЉЕФЕчзгЪНЃК_________________ввЯЉЕФНсЙЙМђЪНЃК____________;
ЃЈ3ЃЉаДГіЯТСаЗДгІЕФЛЏбЇЗНГЬЪНЃКЂй__________________;Ђн______________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПNa2O2ЪЧживЊЕФЛЏЙЄдСЯЃЌОпгаЖржжгУЭОЁЃ
ЃЈ1ЃЉNa2O2ОпгабѕЛЏадЃЌПЩвдНЋSO2бѕЛЏЮЊСђЫсФЦЃЌаДГіИУЗДгІЕФЛЏбЇЗНГЬЪНЃК______________ЃЌИУЗДгІжаЃЌNa2O2ЕФзїгУЮЊ____________ЃЈЬюЁАЛЙдМСЁБЁЂЁАбѕЛЏМСЁБЛђЁАМШЪЧбѕЛЏМСгжЪЧЛЙдМСЁБЃЉЁЃ
ЃЈ2ЃЉNa2O2гыCO2ЗДгІПЩвдВњЩњбѕЦјЁЃФГЭЌбЇЭЈЙ§ЯТСазАжУбщжЄNa2O2ФмЗёгыCO2ЗДгІЁЃ (ЭМжаЬњМмЬЈЕШзАжУвбТдШЅ)ЁЃ
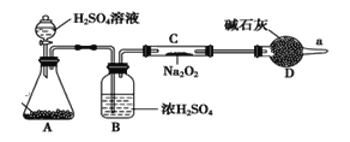
ЂйзАжУAЕФУћГЦЪЧ_______ЃЌAжаЕФЙЬЬхЮЊ_________ЃЌзАжУBжаЪдМСЕФзїгУЮЊ______
ЂкШєNa2O2ФмгыCO2ЃЌдђзАжУCжаЕФЯжЯѓЪЧ____________
ЃЈ3ЃЉЮоЫЎТШЛЏИЦЪЧИЩдяМСЃЌдкaДІЪеМЏЦјЬхЃЌМьВтЗЂЯжИУЦјЬхжаМИКѕЖМЪЧCO2ЦјЬхЃЈЙ§бѕЛЏФЦзуСПЃЉЃЌдђЫЕУїЙ§бѕЛЏФЦгыCO2ЦјЬхВЛЗДгІЁЃИУЭЌбЇВщдФЯрЙиЮФЯзЃЌШЛКѓГЗЕєзАжУBЃЌЦфЫћЖМБЃСєЃЈАќРЈЪдМСЃЉЃЌСЌНгКУзАжУКѓдйДЮНјааЪЕбщЃЌжиаТЪеМЏЦјЬхМьВтЃЌЗЂЯжЕУЕНЕФЦјЬхМИКѕЖМЪЧбѕЦјЃЌИУЪЕбщНсЙћЫЕУїЙ§бѕЛЏФЦгыCO2ЦјЬхЗДгІашвЊ_______________ЁЃ
ЃЈ4ЃЉНЋвЛЖЈСПЕФNa2O2ЙЬЬхЭЖШыЕНКЌгаЯТСаРызгЕФШмвКжаЃКSO32ЃЁЂHCO3-ЁЂCO32ЃЁЂNaЃЋЃЌЗДгІЭъБЯКѓЃЌШмвКжаЩЯЪіРызгЪ§ФПМИКѕВЛБфЕФгаЃЈВЛПМТЧШмвКЬхЛ§ЕФБфЛЏЃЉ__________ЃЈЬюРызгЗћКХЃЉЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЪЕбщНсТле§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
A.ЯђФГШмвКжаМгШыЗгЬЊЃЌШмвКВЛБфЩЋЃЌдђИУШмвКвЛЖЈЯдЫсад
B.ЯђФГШмвКжаМгШыТШЛЏБЕШмвКВњЩњАзЩЋГСЕэЃЌдйМгШыбЮЫсГСЕэВЛЯћЪЇЃЌдђИУШмвКжавЛЖЈга ![]()
C.ЯђФГШмвКжаМгШыЯЁбЮЫсВњЩњЮоЩЋЮоЮЖЦјЬхЃЌНЋЦјЬхЭЈШыГЮЧхЪЏЛвЫЎжаЃЌЪЏЛвЫЎБфЛызЧЃЌдђдШмвКжавЛЖЈга ![]()
D.ЯђФГШмвКжаЕЮМгKSCNЪдМСЃЌШмвКБфГЩбЊКьЩЋЃЌдђИУШмвКжавЛЖЈгаFe3ЃЋ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈNAБэЪОАЂЗќМгЕТТоГЃЪ§ЕФжЕЃЉЃЈЁЁЁЁЃЉ
A.ГЃЮТГЃбЙЯТЃЌ18 g ![]() ЫљКЌЕФЕчзгЪ§ЮЊ10NA
ЫљКЌЕФЕчзгЪ§ЮЊ10NA
B.56 gН№ЪєЬњгыТШЦјЗДгІЪБЕУЕНЕФЕчзгЪ§ЮЊ3NA
C.БъзМзДПіЯТЃЌ22.4 LбѕЦјЫљКЌЕФбѕдзгЪ§ЮЊ2NA
D.ГЃЮТГЃбЙЯТЃЌ22.4 L CO2гызуСПNa2O2ЗДгІзЊвЦЕчзгЪ§ЮЊNA
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЕтЛЏМиЪЧвЛжжЮоЩЋОЇЬхЃЌвзШмгкЫЎЁЃЪЕбщЪвжЦБИKIОЇЬхЕФВНжшШчЯТЃК
Ђё.дкШчЯТЭМЫљЪОЕФШ§ОБЩеЦПжаМгШыбаЯИЕФI2КЭвЛЖЈСПЕФ30%KOHШмвКЃЌНСАшЃЈвбжЊЃКI2гыKOHЗДгІВњЮяжЎвЛЪЧKIO3ЃЉЃЛ
Ђђ.ЕтЭъШЋЗДгІКѓЃЌДђПЊЗжвКТЉЖЗжаЕФЛюШћЁЂЕЏЛЩМа1ЁЂ2ЃЌЯђзАжУCжаЭЈШызуСПЕФH2SЃЛ
Ђѓ.ЗДгІНсЪјКѓЃЌЯђзАжУCжаМгШыЯЁH2SO4ЫсЛЏЃЌЫЎдЁМгШШЃЛ
Ђє.РфШДЃЌЙ§ТЫЕУKIДжШмвКЁЃ
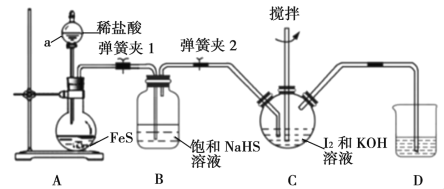
ЃЈ1ЃЉвЧЦїaЕФУћГЦЪЧ__________ЃЌВНжшЂёжаПижЦKOHШмвКЙ§СПЕФФПЕФЪЧ______________ЁЃ
ЃЈ2ЃЉзАжУBЕФзїгУЪЧ_____________ЃЌзАжУDжаЪЂЗХЕФШмвКЪЧ________________ЁЃ
ЃЈ3ЃЉзАжУCжаH2SКЭKIO3ЗДгІЕФРызгЗНГЬЪНЮЊ_______________________ЁЃ
ЃЈ4ЃЉВНжшЂѓжаЫЎдЁМгШШЕФФПЕФЪЧГ§ШЅ_________________________ЃЈЬюЛЏбЇЪНЃЉЁЃ
ЃЈ5ЃЉгЩВНжшЂєЫљЕУЕФKIДжШмвКжаКЌгаЩйСПK2SO4ЃЌашНјааЬсДПЃЌЬсДПСїГЬШчЯТЃК
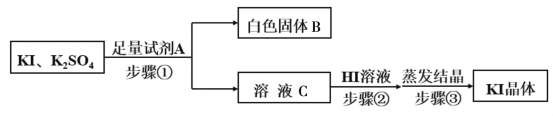
ЂйвбжЊАзЩЋЙЬЬхBЪЧЛьКЯЮяЃЌЪдМСAЮЊ__________ЃЌЮЊГ§ШЅШмвКCжаЕФдгжЪЃЌВНжшЂкжаЕїНкШмвКЮЊШѕЫсадЃЌдђМгШыHIШмвККѓВњЩњЕФЯжЯѓЪЧ___________________ЁЃ
ЂкЮЊВтЖЈзюКѓЫљЕУKIОЇЬхЕФДПЖШЃЌШЁa gОЇЬхХфжЦ100mLШмвКЃЌШЁГі25mLШмвКЃЌЕЮШызуСПЯЁЕФЫсадK2Cr2O7ШмвКЃЌГфЗжЗДгІКѓЃЌЕЮМгМИЕЮЕэЗлШмвКЮЊжИЪОМСЃЌгУb molЁЄLЃ1ЕФNa2S2O3ШмвКНјааЕЮЖЈЃЌЯћКФNa2S2O3ШмвКVmLЁЃ
ЕЮЖЈЙ§ГЬжаЩцМАЕФЗДгІЮЊЃК![]() ЃЌ
ЃЌ![]() дђЕЮЖЈжеЕуЪБЕФЯжЯѓЮЊ________________________ЃЌОЇЬхЕФДПЖШЮЊ_______________________ЃЈСаГіМЦЫуЪНЃЉЁЃ
дђЕЮЖЈжеЕуЪБЕФЯжЯѓЮЊ________________________ЃЌОЇЬхЕФДПЖШЮЊ_______________________ЃЈСаГіМЦЫуЪНЃЉЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПWЪЧгЩAЁЂBСНжждЊЫизщГЩЕФAB2аЭЛЏКЯЮяЁЃ
ЃЈ1ЃЉШєWКЭНЙЬПдкИпЮТЯТЗЂЩњЗДгІЃЌЫљжЦЕУЕФАыЕМЬхВФСЯгІгУЙуЗКЁЃдђWЮЊ________________(ЬюЛЏбЇЪН)ЁЃ
ЃЈ2ЃЉШєШЁСНжЇЪдЙмЗжБ№МгШыЩйСПWШмвКЃЌЭљвЛжЇЪдЙмжаЕЮШыKSCNШмвКЃЌЮоУїЯдЯжЯѓЁЃЭљСэвЛжЇЪдЙмжаМгШызуСПТШЫЎЃЌдйМгШыЪЪСПCCl4ЃЌеёЕДЃЌОВжУЃЌЯТВуШмвКГЪзЯКьЩЋЃЛЭљЩЯВуШмвКжаЕЮШыKSCNШмвКЃЌШмвКГЪКьЩЋЁЃ
ЂйWШмвКжаЫљКЌН№ЪєбєРызгЮЊ________________ЁЃ
ЂкЩЯЪіЪЕбщжаЃЌWгызуСПТШЫЎЗДгІЕФРызгЗНГЬЪНЮЊ_________________________________________________________ЁЃ
ЃЈ3ЃЉШєWЪЧРызгЛЏКЯЮяЃЌЦфвѕЁЂбєРызгОљКЌ18ИіЕчзгЃЌЧввѕЁЂбєРызгИіЪ§БШЮЊ1ЁУ1ЁЃ
ЂйвѕРызгЕФЕчзгЪНЮЊ___________________ЁЃ
Ђк1mol WгызуСПЫЎГфЗжЗДгІЃЌзЊвЦЕчзгЕФЮяжЪЕФСПЮЊ_____________molЁЃ
ЃЈ4ЃЉШєAЁЂBЪЧЭЌжїзхдЊЫиЃЌWШмгкЫЎЩњГЩвЛжжЖўдЊШѕЫсЁЃ
ЂйBдкдЊЫижмЦкБэжаЕФЮЛжУЮЊ______________________________ЁЃ
ЂкШчЙћГЃЮТЯТWЮЊЦјЬхЃЌWЫљаЮГЩЕФЖўдЊШѕЫсШмвКжаЃЌКЌAдЊЫиЕФФГЮЂСЃеМЫљгаКЌAдЊЫиЮЂСЃЕФЮяжЪЕФСПЗжЪ§гыШмвКpHЕФЙиЯЕШчЯТЭМЫљЪОЃЌИУЮЂСЃЕФЛЏбЇЪНЮЊ______________________ЃЛ
ИУЖўдЊШѕЫсЕФвЛМЖЕчРыГЃЪ§ЮЊKa1ЃЌдђpKa1=ЁЊlgKa1Ёж______________________ЁЃ
ЂлШчЙћAдЊЫиЕФжмЦкађЪ§ЪЧBдЊЫиЕФСНБЖЃЌWЕФЫЎШмвКБЛЫЋбѕЫЎбѕЛЏЕФЛЏбЇЗНГЬЪНЮЊ_________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
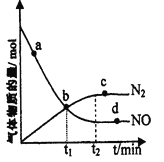
ЁОЬтФПЁПАБЦјПЩзїЮЊЭбЯѕМСЃЌдкКуЮТКуШнУмБеШнЦїжаГфШывЛЖЈСПЕФNOКЭNH3ЃЌдквЛЖЈЬѕМўЯТЗЂЩњЗДгІЃК6NOЃЈgЃЉ+4NH3ЃЈgЃЉ![]() 5N2ЃЈgЃЉ+6H2OЃЈgЃЉЁЃ
5N2ЃЈgЃЉ+6H2OЃЈgЃЉЁЃ
ЃЈ1ЃЉФмЫЕУїИУЗДгІвбДяЕНЦНКтзДЬЌЕФБъжОЪЧ___ЃЈЬюзжФИађКХЃЉ
a.ЗДгІЫйТЪ4vе§ЃЈNH3ЃЉ=5vФцЃЈN2ЃЉ
b.ЕЅЮЛЪБМфРяУПЩњГЩ5mol N2ЃЌЭЌЪБЩњГЩ4mol NH3
c.ШнЦїФкбЙЧПВЛдйЫцЪБМфЖјЗЂЩњБфЛЏ
d.ШнЦїФкn(NOЃЉЃКnЃЈNH3ЃЉЃКnЃЈN2ЃЉЃКnЃЈH2OЃЉ=6ЃК4ЃК5ЃК6
ЃЈ2ЃЉФГДЮЪЕбщжаВтЕУШнЦїФкNOМАN2ЕФЮяжЪЕФСПЫцЪБМфБфЛЏШчЭМЫљЪОЃЌЭМжаvЃЈе§ЃЉгыvЃЈФцЃЉЯрЕШЕФЕуЮЊ____ЃЈбЁЬюзжФИЃЉЁЃ
ЃЈ3ЃЉвЛЖЈЬѕМўЯТЃЌдк2LУмБеШнЦїФкЃЌЗДгІ2NO2![]() N2O4ЃЌnЃЈNO2ЃЉЫцЪБМфБфЛЏШчБэЃК
N2O4ЃЌnЃЈNO2ЃЉЫцЪБМфБфЛЏШчБэЃК
ЪБМф/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO2)/mol | 0.040 | 0.020 | 0.010 | 0.005 | 0.005 | 0.005 |
гУNO2БэЪО0~2sФкИУЗДгІЕФЦНОљЗДгІЫйТЪ____ЁЃдкЕк5sЪБЃЌNO2ЕФзЊЛЏТЪЮЊ____ЁЃИљОнБэжаПЩвдПДГіЃЌЫцзХЗДгІНјааЃЌЗДгІЫйТЪж№НЅМѕаЁЃЌЦфдвђЪЧ___ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПCO2ЪЧздШЛНчЬМбЛЗжаЕФживЊЮяжЪЁЃЯТСаЙ§ГЬЛсв§Ц№ДѓЦјжаCO2КЌСПЩЯЩ§ЕФЪЧ
A. ЙтКЯзїгУ B. здШЛНЕгъ
C. ЛЏЪЏШМСЯЕФШМЩе D. ЬМЫсбЮЕФГСЛ§
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com