
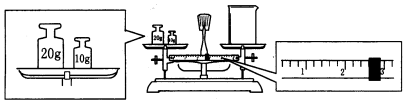
���� ��1������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ����ǩ���ݴ�������������һ�����ʵ���Ũ����Һ��һ�㲽��ѡ����Ҫ������
��2����ƽ���������⣬�ȿ�ͼ�г�����ʽ�Ƿ����������룬������ǣ�����Ϊ��������룬�پ�ͼ����������m=CVM������Ҫ���ʵ�������
��3������ƿ���л�����ʹ�ù�������Ҫ���µߵ���Ϊ��ֹ©ˮ��ʹ��ǰӦ����Ƿ�©ˮ��
��4���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=$\frac{n}{V}$�����жϣ�
��� �⣺��1������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ����ǩ��������ȷ�IJ���Ϊ���ڢ٢ۢ�ݢޢ�ߢܣ��õ���������������ƽ��Կ�ס��ձ���������������ƿ����ͷ�ιܣ�����225mL��ҺӦѡ��250mL����ƿ�����Ի�ȱ�ٵ�������250 mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ���ڢ٢ۢ�ݢޢ�ߢܣ�250 mL����ƿ����ͷ�ιܣ�
��2���ȿ�ͼ�г�����ʽ���������������Ʒ����Ϊ����-���룬�پ�ͼ����������20��10��30g������2.6g�������ձ�����Ϊ10+20-2.6=27.4g������0.5mol/L��NaOH��Һ225mL��Ӧѡ��250mL����ƿ����ȡ���ʵ�����Ϊ��0.5mol/L��0.25L��40g/mol=5.0g��
�ʴ�Ϊ��27.4��5.0��
��3������ƿ���л�����ʹ�ù�������Ҫ���µߵ���Ϊ��ֹ©ˮ��ʹ��ǰӦ����Ƿ�©ˮ��
�ʴ�Ϊ����©��
��4����û��ϴ���ձ��Ͳ��������������ʲ�����ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ��ʲ�ѡ��
��ת����Һʱ������������������ƿ���棬�������ʲ�����ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ��ʲ�ѡ��
�۶���ʱ���ӿ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���ѡ��
������ƿ�����������������ˮ�������ʵ����ʵ�������Һ������������Ӱ�죬��ҺŨ�Ȳ��䣬�ʲ�ѡ��
�ݶ��ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ��ʲ�ѡ��
��ѡ���ۣ�
���� ������Ҫ������һ�����ʵ���Ũ�ȵ����Ʋ��衢����������������ȷ����ԭ�������������ǽ���ؼ���ע��������������ע������ƿ��������ƽʹ�÷�����


 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶��ϵ�һ��ѧ�ο��Ի�ѧ���������棩 ���ͣ�ѡ����
���й����Ȼ�ѧ��Ӧ����������ȷ����( ��
A��HCl��NaOH��Ӧ�к��Ȧ�H����57.3 kJ��mol��1��H2SO4��Ba(OH)2��Ӧ�Ȧ�H��2��(��57.3)kJ��mol��1
B��1 mol����ȼ��������̬ˮ�Ͷ�����̼�������ų����������Ǽ����ȼ����
C��CO(g)��ȼ������283.0 kJ��mol��1����2CO2(g) ��2CO(g)��O2(g)��Ӧ�Ħ�H��+2��283.0 kJ��mol��1
D����Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶���10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���ʵ���Ũ����ͬ��������Һ�У�NH4+Ũ�������� ( )
A��NH4Cl B�� NH4HSO4 C��CH3COONH4 D��NH4HCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
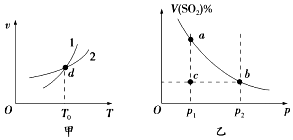
| A�� | ͼ���У�����1��ʾ�淴Ӧ�������¶ȵĹ�ϵ | |
| B�� | ͼ���У�d��ʱ����������ƽ��Ħ���������ٸı� | |
| C�� | ͼ���У�a��b����ķ�Ӧ���ʣ�va��vb | |
| D�� | ͼ���У�c��������淴Ӧ���ʣ�v���棩��v������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | a=b=c | B�� | b��a��c | C�� | b��c��a | D�� | b=c��a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶���10���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��pH��ֽ�ⶨij��ɫ��Һ��pHʱ���淶�IJ����ǣ�  ��
��
A����pH��ֽ������Һ�й۲�����ɫ�仯��������ɫ���Ƚ�
B������Һ����pH��ֽ�ϣ�������ɫ���Ƚ�
C���ø���Ľྻ������պȡ��Һ������pH��ֽ�ϣ�������ɫ���Ƚ�
D�����Թ��ڷ���������Һ����У���pH��ֽ���ڹܿڣ��۲���ɫ��������ɫ���Ƚ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 5Cl2+I2+6H2O�T10HCl+2HIO3 | |
| B�� | 2Cl2+2Ca��OH��2�TCaCl2+Ca��ClO��+2H2O | |
| C�� | MnO2+4HCI$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+2H2O+Cl2�� | |
| D�� | 2NaCl+2H2O $\frac{\underline{\;���\;}}{\;}$2NaOH++Cl2��+H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| t�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 1.7 | 1.1 | 1.0 | 0.6 | 0.4 |
| ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| H2O | CO | CO2 | CO | |||
| A | 650 | 2 | 4 | 1.6 | 2.4 | 5 |
| B | 900 | 1 | 2 | 0.4 | 1.6 | 3 |
| C | 900 | a | b | c | d | t |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com