
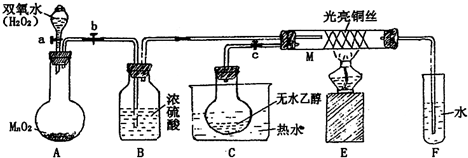
ÓŠ¹Ų“߻ƼĮµÄ“߻ƻśĄķµČĪŹĢāæÉŅŌ“Ó”°ŅŅ“¼“ß»ÆŃõ»ÆŹµŃé”±µĆµ½Ņ»Š©ČĻŹ¶£¬Ä³½ĢŹ¦Éč¼ĘĮĖČēĶ¼×°ÖĆ£Ø¼Š³Ö×°ÖĆŅ»ĘšŅŃŹ”ĀŌ£©£¬Ę䏵Ńé²Ł×÷ĪŖ£ŗĻČ°“Ķ¼°²×°ŗĆ£¬ĻČ¹Ų±Õ»īČūa”¢b”¢c£¬ŌŚĶĖæµÄÖŠ¼ä²æ·Ö¼ÓČČʬæĢ£¬Č»ŗó“ņæŖ»īČūa”¢b”¢c£¬ĶعżæŲÖĘ»īČūbŗĶc£¬¶ųÓŠ½Ś×ą£Ø¼äŠŖŠŌ£©ĶØČėĘųĢ壬¼“æÉŌŚM“¦¹Ū²ģµ½Ć÷ĻŌµÄŹµŃéĻÖĻó”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ

£Ø£±£©CÖŠČČĖ®µÄ×÷ÓĆ£ŗ””””””””””””””””””””””””””””””””””””””””””””””””””””””””””
£Ø£²£©£Ķ“¦·¢ÉśµÄ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ””””””””””””””””””””””””””””””””””
£Ø£³£©“Ó£ĶÖŠæɹŪ²ģµ½µÄĻÖĻó£ŗ””””””””””””””””””””””””””””””””””””””””””””””
“ÓÖŠæÉČĻŹ¶µ½øĆŹµŃé¹ż³ĢÖŠ“߻ƼĮ””””””£ØĢī”°²Ī¼Ó”±»ņ”°²»²Ī¼Ó”±£©»Æѧ·“Ó¦£¬»¹æÉŅŌČĻŹ¶µ½“ß»Æ×÷ÓĆŠčŅŖŅ»¶ØµÄ
£Ø4£©ŹµŃéŅ»¶ĪŹ±¼äŗó£¬Čē¹ū³·µō¾Ę¾«µĘ£¬·“Ó¦ £ØĢī”°ÄÜ”±»ņ”°²»ÄÜ”±£©¼ĢŠų½ųŠŠ£¬ĘäŌŅņŹĒ””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””””
£Ø£±£©CÖŠČČĖ®Ź¹ŅŅ“¼Ęų»ÆµĆµ½ŅŅ“¼ÕōĘų
£Ø£²£©![]() £Ø£³£©Ėę׿äŠŖŠŌµÄĶØČėŃõĘų£¬æÉŅŌ擵½ŹÜČČ²æ·ÖµÄŃõ»ÆĶ½»Ģę³öĻÖ±äŗŚ”¢±äŗģµÄĻÖĻó£»²Ī¼Ó”¢ĪĀ¶Č£Ø4£©ÄÜ£»ÓÉÓŚŅŅ“¼“ß»ÆŃõ»ÆŹĒ·ÅČČ·“Ó¦£¬µ±³·µō¾Ę¾«µĘŗó£¬ŌŚ·“Ó¦·Å³öČČĮæµÄ×÷ÓĆĻĀ£¬ČŌÄÜŹ¹·“Ó¦¼ĢŠų½ųŠŠ”£
£Ø£³£©Ėę׿äŠŖŠŌµÄĶØČėŃõĘų£¬æÉŅŌ擵½ŹÜČČ²æ·ÖµÄŃõ»ÆĶ½»Ģę³öĻÖ±äŗŚ”¢±äŗģµÄĻÖĻó£»²Ī¼Ó”¢ĪĀ¶Č£Ø4£©ÄÜ£»ÓÉÓŚŅŅ“¼“ß»ÆŃõ»ÆŹĒ·ÅČČ·“Ó¦£¬µ±³·µō¾Ę¾«µĘŗó£¬ŌŚ·“Ó¦·Å³öČČĮæµÄ×÷ÓĆĻĀ£¬ČŌÄÜŹ¹·“Ó¦¼ĢŠų½ųŠŠ”£
±¾ĢāŅŌæĪ±¾ŹµŃéĪŖ»ł“”£¬æ¼²éѧɜµÄŹµŃéÄÜĮ¦£»±¾ĢāĮ¢×ć½Ģ²Ä£¬µ«øßÓŚ½Ģ²Ä£¬ŹµŃéµÄÄæµÄŹĒĪŖĮĖŃéÖ¤ŅŅ“¼µÄ“ß»ÆŃõ»Æ£¬¼ģŃéĘä²śĪļ¼°“߻ƼĮµÄŠŌÖŹ£¬ÓƵÄŅ©Ę·ĪŖŅŅ“¼”¢ŃõĘųŗĶŃõ»ÆĶ£¬×°ÖĆAŹĒÖĘČ”ŃõĘųµÄ×°ÖĆ£¬·“Ó¦µÄ·½³ĢŹ½ĪŖ£ŗ2H2O2![]() 2H2O£«O2”ü£»ĪŖĮĖµĆµ½øÉŌļµÄŃõĘų£¬Éś³ÉµÄĘųĢåĶØČėBÖŠ”£ŌŚC×°ÖĆÖŠ¶ŌĪŽĖ®ŅŅ“¼½ųŠŠĖ®Ō”¼ÓČČ£¬µĆµ½ŅŅ“¼ÕōĘų½ųČė×°ÖĆMÖŠ£¬ŌŚŃõ»ÆĶµÄ×÷ÓĆĻĀ½ųŠŠ“ß»ÆŃõ»Æ·“Ó¦£¬·“Ó¦µÄ·½³ĢŹ½ĪŖ£ŗ
2H2O£«O2”ü£»ĪŖĮĖµĆµ½øÉŌļµÄŃõĘų£¬Éś³ÉµÄĘųĢåĶØČėBÖŠ”£ŌŚC×°ÖĆÖŠ¶ŌĪŽĖ®ŅŅ“¼½ųŠŠĖ®Ō”¼ÓČČ£¬µĆµ½ŅŅ“¼ÕōĘų½ųČė×°ÖĆMÖŠ£¬ŌŚŃõ»ÆĶµÄ×÷ÓĆĻĀ½ųŠŠ“ß»ÆŃõ»Æ·“Ó¦£¬·“Ó¦µÄ·½³ĢŹ½ĪŖ£ŗ![]() £»Ėę׿äŠŖŠŌµÄĶØČėŃõĘų£¬æÉŅŌ擵½ŹÜČČ²æ·ÖµÄŃõ»ÆĶ½»Ģę³öĻÖ±äŗŚ”¢±äŗģµÄĻÖĻ󣬶ųĪ“¼ÓČČ²æ·ÖµÄŃõ»ÆĶ²»±ä£¬ĖµĆ÷ŌŚŹµŃé¹ż³ĢÖŠ£¬“߻ƼĮĮĖ»Æѧ·“Ó¦£¬¶ųĒŅŠčŅŖŅ»¶ØµÄĪĀ¶Č²ÅÄÜĘšµ½“ß»Æ×÷ÓĆ”£µ±ÓÉÓŚŅŅ“¼“ß»ÆŃõ»ÆŹĒ·ÅČČ·“Ó¦£¬µ±³·µō¾Ę¾«µĘŗó£¬ŌŚ·“Ó¦·Å³öČČĮæµÄ×÷ÓĆĻĀ£¬ČŌÄÜŹ¹·“Ó¦¼ĢŠų½ųŠŠ”£
£»Ėę׿äŠŖŠŌµÄĶØČėŃõĘų£¬æÉŅŌ擵½ŹÜČČ²æ·ÖµÄŃõ»ÆĶ½»Ģę³öĻÖ±äŗŚ”¢±äŗģµÄĻÖĻ󣬶ųĪ“¼ÓČČ²æ·ÖµÄŃõ»ÆĶ²»±ä£¬ĖµĆ÷ŌŚŹµŃé¹ż³ĢÖŠ£¬“߻ƼĮĮĖ»Æѧ·“Ó¦£¬¶ųĒŅŠčŅŖŅ»¶ØµÄĪĀ¶Č²ÅÄÜĘšµ½“ß»Æ×÷ÓĆ”£µ±ÓÉÓŚŅŅ“¼“ß»ÆŃõ»ÆŹĒ·ÅČČ·“Ó¦£¬µ±³·µō¾Ę¾«µĘŗó£¬ŌŚ·“Ó¦·Å³öČČĮæµÄ×÷ÓĆĻĀ£¬ČŌÄÜŹ¹·“Ó¦¼ĢŠų½ųŠŠ”£


 Ģ½¾æÓė¹®¹ĢŗÓÄĻæĘѧ¼¼Źõ³ö°ęÉēĻµĮŠ“š°ø
Ģ½¾æÓė¹®¹ĢŗÓÄĻæĘѧ¼¼Źõ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
| Cu |
| ”÷ |
| Cu |
| ”÷ |
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ||
| ||
| Cu |
| ”÷ |
| Cu |
| ”÷ |
| ”÷ |
| ”÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010-2011ѧğŌĘÄĻŹ”Īä¶ØŅ»ÖŠø߶žĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
£Ø7·Ö£©ÓŠ¹Ų“߻ƼĮµÄ“߻ƻśĄķĪŹĢāĪŅĆĒæÉŅŌ“Ó”°ŅŅ“¼“ß»ÆŹµŃé”±ÖŠµĆµ½Ņ»Š©ČĻŹ¶”£Ä³½ĢŹ¦Éč¼ĘĮĖĻĀĶ¼ĖłŹ¾×°ÖĆ£Ø¼Š³Ö×°ÖĆŅŃŹ”ĀŌ£©£¬ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©AÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ___________________________ £»
£Ø2£©·“Ó¦Ņ»¶ĪŹ±¼äŗó£¬ČōŅĘČ„¾Ę¾«µĘ·“Ó¦ČŌÄܼĢŠų½ųŠŠ£¬ŌņĖµĆ÷øĆŅŅ“¼µÄŃõ»Æ·“Ó¦ŹĒ_________________(Ģī”°·¢ČČ”±»ņ”°ĪüČČ”±)·“Ó¦£»
£Ø3£©ŹµŃé¹ż³ĢÖŠD“¦ĶĖæĶųÓŠŗģÉ«ŗĶŗŚÉ«½»Ģę³öĻÖµÄĻÖĻó£¬ĒėÓĆ»Æѧ·½³ĢŹ½½āŹĶŌŅņ”£ĻÖĻó¢Ł£ŗŗģÉ«±äŗŚ É«£ŗ___________________________________________£»
É«£ŗ___________________________________________£»
ĻÖĻó¢Ś£ŗŗŚÉ«±äŗģÉ«£ŗ____________________________________________
“ÓÕāŠ©ŹµŃéĻÖĻóÖŠæÉŅŌČĻŹ¶µ½ŹµŃé¹ż³ĢÖŠ“߻ƼĮ__________£ØĢī”°²Ī¼Ó”±»ņ”°²»²Ī¼Ó”±£©»Æѧ·“Ó¦”£
£Ø4£©×°ÖĆB”¢FµÄ×÷ÓĆ·Ö±šŹĒ
B:______________________________________ ,
F:_________________________________ ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģŗŚĮś½Ź”ø߶žĻĀѧʌµŚŅ»“Ī¼ģ²ā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ÓŠ¹Ų“߻ƼĮµÄ“߻ƻśĄķµČĪŹĢāæÉŅŌ“Ó”°ŅŅ“¼“ß»ÆŃõ»ÆŹµŃé”±µĆµ½Ņ»Š©ČĻŹ¶£¬Ä³½ĢŹ¦Éč¼ĘĮĖČēĶ¼×°ÖĆ£Ø¼Š³Ö×°ÖĆŅĒĘ÷ŅŃŹ”ĀŌ£©£¬Ę䏵Ńé²Ł×÷ĪŖ£ŗ°“Ķ¼°²×°ŗĆ×°ÖĆ£¬ĻČ¹Ų±Õ»īČūa”¢b”¢c£¬ŌŚĶĖæµÄÖŠ¼ä²æ·Ö¼ÓČČʬæĢ£¬Č»ŗó“ņæŖ»īČūa”¢b”¢c£¬ĶعżæŲÖĘ»īČūaŗĶb£¬¶ųÓŠ½Ś×ą£Ø¼äŠŖŠŌ£©ĶØČėĘųĢ壬¼“æÉŌŚM“¦¹Ū²ģµ½Ć÷ĻŌµÄŹµŃéĻÖĻó”£

£Ø1£©AÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ_____________£¬BµÄ×÷ÓĆ£ŗ____________£» CµÄ×÷ÓĆ£ŗ____________”£
£Ø2£©M“¦·¢ÉśµÄ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ______________________________________
£Ø3£©“ÓM¹ÜÖŠæɹŪ²ģµ½µÄĻÖĻóĪŖ_______________£»“ÓÖŠæÉČĻŹ¶µ½øĆŹµŃé¹ż³ĢÖŠ“߻ƼĮ___ __ ____£ØĢī”°²Ī¼Ó”±»ņ”°²»²Ī¼Ó”±£©ĮĖ»Æѧ·“Ó¦
£Ø4£©ŃéÖ¤ŅŅ“¼Ńõ»Æ²śĪļµÄŹŌ¼ĮŹĒ £¬²¢Š“³ö¶ŌÓ¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø5£©ČōŹŌ¹ÜFÖŠŹÕ¼Æµ½µÄŅŗĢåÓĆ×ĻÉ«ŹÆČļŹŌÖ½¼ģŃ飬ŹŌÖ½ĻŌŗģÉ«£¬ĖµĆ÷ŅŗĢåÖŠ»¹ŗ¬ÓŠ ”£ŅŖ³żČ„øĆĪļÖŹ£¬æÉĻÖŌŚ»ģŗĻŅŗÖŠ¼ÓČė £ØĢīŠ“×ÖÄø£©”£
A£®ĀČ»ÆÄĘČÜŅŗ B£®±½ C£®Ģ¼ĖįĒāÄĘČÜŅŗ D£®ĖÄĀČ»ÆĢ¼
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģŌĘÄĻŹ”ø߶žĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
£Ø7·Ö£©ÓŠ¹Ų“߻ƼĮµÄ“߻ƻśĄķĪŹĢāĪŅĆĒæÉŅŌ“Ó”°ŅŅ“¼“ß»ÆŹµŃé”±ÖŠµĆµ½Ņ»Š©ČĻŹ¶”£Ä³½ĢŹ¦Éč¼ĘĮĖĻĀĶ¼ĖłŹ¾×°ÖĆ£Ø¼Š³Ö×°ÖĆŅŃŹ”ĀŌ£©£¬ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ

£Ø1£©AÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ___________________________ £»
£Ø2£©·“Ó¦Ņ»¶ĪŹ±¼äŗó£¬ČōŅĘČ„¾Ę¾«µĘ·“Ó¦ČŌÄܼĢŠų½ųŠŠ£¬ŌņĖµĆ÷øĆŅŅ“¼µÄŃõ»Æ·“Ó¦ŹĒ_________________(Ģī”°·¢ČČ”±»ņ”°ĪüČČ”±)·“Ó¦£»
£Ø3£©ŹµŃé¹ż³ĢÖŠD“¦ĶĖæĶųÓŠŗģÉ«ŗĶŗŚÉ«½»Ģę³öĻÖµÄĻÖĻó£¬ĒėÓĆ»Æѧ·½³ĢŹ½½āŹĶŌŅņ”£ĻÖĻó¢Ł£ŗŗģÉ«±äŗŚÉ«£ŗ___________________________________________£»
ĻÖĻó¢Ś£ŗŗŚÉ«±äŗģÉ«£ŗ____________________________________________
“ÓÕāŠ©ŹµŃéĻÖĻóÖŠæÉŅŌČĻŹ¶µ½ŹµŃé¹ż³ĢÖŠ“߻ƼĮ__________£ØĢī”°²Ī¼Ó”±»ņ”°²»²Ī¼Ó”±£©»Æѧ·“Ó¦”£
£Ø4£©×°ÖĆB”¢FµÄ×÷ÓĆ·Ö±šŹĒ
B:______________________________________ ,
F:_________________________________ ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com