



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
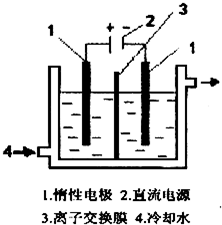 ����أ�KlO3���������ҹ��涨��ʳ�μӵ������ ͨ�����Ե�Ϊԭ�������KOH��Һͨ�����з�Ӧ�� �ã�3I2+6K0H=5KI+KI03+3H20���ٽ� KI �� KIO3 �Ļ� ����Һ��⣬�����е�I-ת��ΪIO-3��װ����ͼ��
����أ�KlO3���������ҹ��涨��ʳ�μӵ������ ͨ�����Ե�Ϊԭ�������KOH��Һͨ�����з�Ӧ�� �ã�3I2+6K0H=5KI+KI03+3H20���ٽ� KI �� KIO3 �Ļ� ����Һ��⣬�����е�I-ת��ΪIO-3��װ����ͼ��| ʵ����� | ������ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
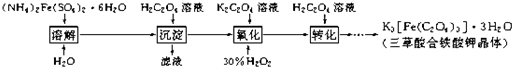
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
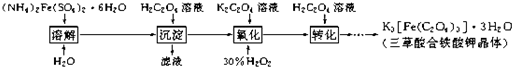
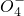 ����ԭ��Mn2+����
����ԭ��Mn2+�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com