
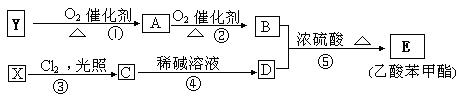
 ��
�� ����
���� ��
�� ��
��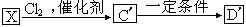
 ��
��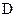 �ķ���ʽ��ͬ����
�ķ���ʽ��ͬ���� ����
����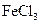 ��Һ������ɫ��Ӧ��д��������������X��
��Һ������ɫ��Ӧ��д��������������X��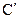 (
(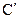 ��һ����������2��)�ķ�Ӧ����ʽ_______ ��
��һ����������2��)�ķ�Ӧ����ʽ_______ �� (2��)
(2��) (1��)
(1��)
 ��2�֣�
��2�֣� (2��)
(2��)


 ����
����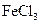 ��Һ������ɫ��Ӧ˵���ǻ����ڱ����ϣ�����C����ԭ�����ڱ����ϡ�
��Һ������ɫ��Ӧ˵���ǻ����ڱ����ϣ�����C����ԭ�����ڱ����ϡ� ��һ����������2�֣�˵��C�����ڱ���������ȡ�������ڶ�λ�����ԣ�X��
��һ����������2�֣�˵��C�����ڱ���������ȡ�������ڶ�λ�����ԣ�X��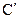 �ķ�Ӧ����ʽ��
�ķ�Ӧ����ʽ�� ��
��

 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
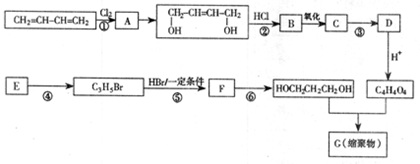
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
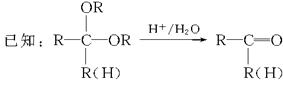
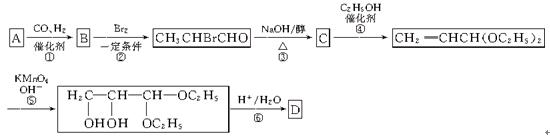
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
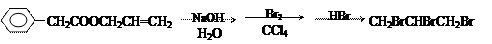
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
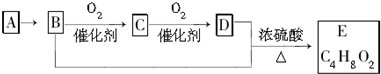
| A������A�ͼ����ѡ��ʹ�����Ը��������Һ | |||
| B��D�к��еĹ�����Ϊ�Ȼ�������D���ʿ������ˮ���е�ˮ�� | |||
| C������C�Ľṹ��ʽΪCH3CHO��E������Ϊ�������� | |||
D��B+D��E�Ļ�ѧ����ʽΪ��CH3CH2OH+CH3COOH
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ����������һ�����������������ᣨHNO2����Ӧ�õ�
����������һ�����������������ᣨHNO2����Ӧ�õ� ���ǻ��ᡣ
���ǻ��ᡣ ���Ը�����ͼ��ʾ��ת����ϵ�ش���
���Ը�����ͼ��ʾ��ת����ϵ�ش���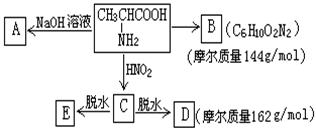
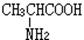 ��
��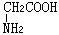 �������һ��Ӧ�����У�һ�������·������ķ�Ӧ����������� �ֶ��ġ�
�������һ��Ӧ�����У�һ�������·������ķ�Ӧ����������� �ֶ��ġ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
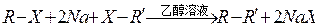

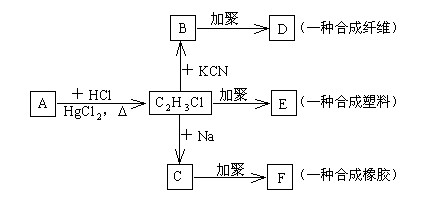
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A����ϩ���Ҷ�����CH2==CH2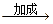 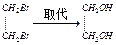 |
B����������Ҵ���CH3CH2Br CH2==CH2 CH2==CH2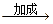 CH3CH2OH CH3CH2OH |
C����ȩ����ϩ��CH3CHO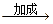 CH3CH2OH CH3CH2OH CH2==CH2 CH2==CH2 |
D���Ҵ������CH3CH2OH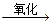 CH3CHO CH3CHO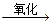 CH3COOH CH3COOH |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com