
����������������Ʒ��������̨������Ȧ����ʽ���У� ����ƿ ����ʽ�ζ��ܺͼ�ʽ�ζ��� ���ձ������ɣ� �ݲ����� ��ͷ�ι� ����ƽ�������룩 ����ֽ ����Ͳ �����©����
����ҩƷ����A��NaOH���� ��B����NaOH��Һ ��C��δ֪Ũ������ ��D������ˮ��E��̼������Һ
������������ѧ��ʵ�飬�ش��������⣺
��1������ʱ��Ӧѡ�õ����������� �����ţ���
��2������250mlһ�����ʵ���Ũ�ȵ�NaOH��Һʱ����ȱ�ٵ������� ��
��3��������к͵ζ�ʱ����ȱ�����Լ��� ��
��4�������к͵ζ�ʱ���������ý�Ҫʢ����Һ������ϴ�����������е� �����ţ���
�ף���ʽ�ζ��� �ң���ʽ�ζ��� ����25 mL��Ͳ ������ƿ
��5��ijͬѧ��һ����֪Ũ�ȵ�������Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ�����в����Եζ��Ľ����ʲôӰ�죿���ƫ����ƫС������Ӱ�족��
����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ��_____________
�ڵζ�ǰ���ζ��ܼ�������ݣ��ζ���������ʧ��_____________
�۶���ʽ�ζ��ܵĿ̶�ʱ���ζ�ǰ���Ӱ�Һ����ʹ����ζ����Ӷ�����_______
�ܵζ����յ㸽��ʱ������ƿ�м�����������ˮ��ϴƿ����մ����Һ_____
��1���٢ܢݢ�⡣��2��250ml������ƿ��
��3�����ָʾ��(��̪�����)��
��4������5����ƫ���ƫ���ƫС����Ӱ��
��������
�����������1��������Ҫ�õ������У�����̨������Ȧ����ʽ���У����ձ�������������ֽ������©����
��2������250mlһ�����ʵ���Ũ�ȵ�NaOH��Һ��Ҫ250mL����ƿ��
��3����ȷ���жϵζ��յ���Լ������ָʾ����
��4�������ϴ��ƿ����ʹ��ƿ����ȡ��Һ���࣬�����
��5������ʽ�ζ���δ�ñ�������ϴ����ʹ�������Ũ�ȱ�С����ɵζ����ƫ��
�ڵζ�ǰ���ζ��ܼ�������ݣ��ζ���������ʧ��˵���������������ֵ����ʵ��ֵ�����ƫ��
�۵ζ�ǰ���Ӱ�Һ����ʹ����ζ����Ӷ�������Һ�IJ���ֵ��С�����ƫС��
������ƿ�м�����������ˮ��ϴƿ����մ����Һ����Ӱ���������������Եζ������Ӱ�졣
���㣺���⿼�����������ѡ������һ�����ʵ���Ũ����Һ���ۺϵζ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��I�����в����ᵼ��ʵ����ƫ�ߵ���
��I�����в����ᵼ��ʵ����ƫ�ߵ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1�����в����ᵼ��ʵ����ƫ�ߵ���
A������һ�����ʵ���Ũ�ȵ�������Һʱ������ҡ�Ⱥ���Һ����ڿ̶��ߡ�
?B��������һ�����ʵ���Ũ����Һʱ���� 10 mL����Ͳ��ȡ5.0 mLҺ������ʱ���Ӷ���
?C������ƽ����20.5gij���ʣ������ҩƷ��λ�÷ŷ�������ҩƷ������
?D��10%�������90%�����������������50����������Һ
?E������һ�����ʵ���Ũ����Һʱ������ʱ���Ӷ�����������Һ��Ũ��
?��������������������Ʒ��a������̨������Ȧ�����У� b����ƿc.�ζ��� d���ձ������ɣ� e�������� f.��ͷ�ι� g��������ƽ�������룩 h����ֽ i����Ͳ j��©�� k���¶ȼơ�
? ���������Լ���A��NaOH���� B��̼������Һ C���Ȼ�þ��Һ D������ˮ������գ�
?��1����ͼ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣������жϼ����������߿̶ȣ�����ȷ����
��
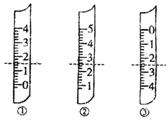 ?A������������Ϊ1.5mL
?A������������Ϊ1.5mL
?B��������Ͳ������Ϊ2.5mL
?C�����ǵζ��ܣ�����Ϊ2.50mL
?D�������¶ȼƣ�������2.5��
?��2������һ�����ʵ���Ũ�ȵ�����������Һʱ����ȱ�ٵ�������
?��3����ȥ Mg��OH��2�л��е����� Ca��OH��2�������õ��Լ��ǣ� ��ѡ����ţ������������� �� ϴ�ӡ��������������������������Ʒ�õ����� ����������������Ʒ��ѡ��������Ӧ��ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1�����в����ᵼ��ʵ����ƫ�ߵ���
A������һ�����ʵ���Ũ�ȵ�������Һʱ������ҡ�Ⱥ���Һ����ڿ̶��ߡ�
B��������һ�����ʵ���Ũ����Һʱ���� 10 mL����Ͳ��ȡ5.0 mLҺ������ʱ���Ӷ���
C������ƽ����20.5gij���ʣ������ҩƷ��λ�÷ŷ�������ҩƷ������
D��10%�������90%�����������������50����������Һ
E������һ�����ʵ���Ũ����Һʱ������ʱ���Ӷ�����������Һ��Ũ��
?��������������������Ʒ��a������̨������Ȧ�����У� b����ƿc.�ζ��� d���ձ������ɣ� e�������� f.��ͷ�ι� g��������ƽ�������룩 h����ֽ i����Ͳ j��©�� k���¶ȼơ�
? ���������Լ���A��NaOH���� B��̼������Һ C���Ȼ�þ��Һ D������ˮ������գ�
?��1����ͼ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣������жϼ����������߿̶ȣ�����ȷ���� ��

? A������������Ϊ1.5mL
? B��������Ͳ������Ϊ2.5mL
? C�����ǵζ��ܣ�����Ϊ2.50mL
? D�������¶ȼƣ�������2.5��
?��2������һ�����ʵ���Ũ�ȵ�����������Һʱ����ȱ�ٵ�������
?��3����ȥ Mg��OH��2�л��е����� Ca��OH��2�������õ��Լ��ǣ� ��ѡ����ţ������������� �� ϴ�ӡ��������������������������Ʒ�õ����� ����������������Ʒ��ѡ��������Ӧ��ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1�����в����ᵼ��ʵ����ƫ�ߵ���
A������һ�����ʵ���Ũ�ȵ�������Һʱ������ҡ�Ⱥ���Һ����ڿ̶��ߡ�
B��������һ�����ʵ���Ũ����Һʱ���� 10 mL����Ͳ��ȡ5.0 mLҺ������ʱ���Ӷ���
C������ƽ����20.5gij���ʣ������ҩƷ��λ�÷ŷ�������ҩƷ������
D��10%�������90%�����������������50����������Һ
E������һ�����ʵ���Ũ����Һʱ������ʱ���Ӷ�����������Һ��Ũ��
?��������������������Ʒ��a������̨������Ȧ�����У� b����ƿc.�ζ��� d���ձ������ɣ� e�������� f.��ͷ�ι� g��������ƽ�������룩 h����ֽ i����Ͳ j��©�� k���¶ȼơ�
? ���������Լ���A��NaOH���� B��̼������Һ C���Ȼ�þ��Һ D������ˮ������գ�
?��1����ͼ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣������жϼ����������߿̶ȣ�����ȷ���� ��
? A������������Ϊ1.5mL
? B��������Ͳ������Ϊ2. 5mL
? C�����ǵζ��ܣ�����Ϊ2.50mL
? D�������¶ȼƣ�������2.5��
?��2������һ�����ʵ���Ũ�ȵ�����������Һʱ����ȱ�ٵ�������
?��3����ȥ Mg��OH��2�л��е����� Ca��OH��2�������õ��Լ��ǣ� ��ѡ����ţ������������� �� ϴ�ӡ��������������������������Ʒ�õ����� ����������������Ʒ��ѡ��������Ӧ��ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1�����в����ᵼ��ʵ����ƫ�ߵ���
A������һ�����ʵ���Ũ�ȵ�������Һʱ������ҡ�Ⱥ���Һ����ڿ̶��ߡ�
?B��������һ�����ʵ���Ũ����Һʱ���� 10 mL����Ͳ��ȡ5.0 mLҺ������ʱ���Ӷ���
?C������ƽ����20.5gij���ʣ������ҩƷ��λ�÷ŷ�������ҩƷ������
?D��10%�������90%�����������������50����������Һ
?E������һ�����ʵ���Ũ����Һʱ������ʱ���Ӷ�����������Һ��Ũ��
?��������������������Ʒ��a������̨������Ȧ�����У� b����ƿc.�ζ��� d���ձ������ɣ� e�������� f.��ͷ�ι� g��������ƽ�������룩 h����ֽ i����Ͳ j��©�� k���¶ȼơ�
? ���������Լ���A��NaOH���� B��̼������Һ C���Ȼ�þ��Һ D������ˮ������գ�
?��1����ͼ�ֱ����¶ȼơ���Ͳ���ζ��ܵ�һ���֣������жϼ����������߿̶ȣ�����ȷ����
��
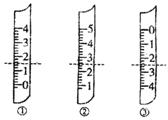 ?A������������Ϊ1.5mL
?A������������Ϊ1.5mL
?B��������Ͳ������Ϊ2.5mL
?C�����ǵζ��ܣ�����Ϊ2.50mL
?D�������¶ȼƣ�������2.5��
?��2������һ�����ʵ���Ũ�ȵ�����������Һʱ����ȱ�ٵ�������
?��3����ȥ Mg��OH��2�л��е����� Ca��OH��2�������õ��Լ��ǣ� ��ѡ����ţ������������� �� ϴ�ӡ��������������������������Ʒ�õ����� ����������������Ʒ��ѡ��������Ӧ��ţ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com