
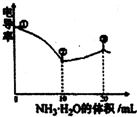
| A���ٵ���Һ��C��H+��Ϊ0.200 mol/L |
| B����Һ�¶��ڢ�ʱ��� |
| C���۵���Һ����c��C1-����c��CH3COO-�� |
| D���۵����������Ŀ������ʹ��Һ�絼���Խ��� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
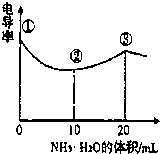 ��2013?����һģ���絼�ʿ����ں����������Һ����������С���ҵ絼��Խ����Һ�ĵ�������Խǿ�������£���0.100mol?L-1��NH3?H2O �ζ�10.00mL Ũ�Ⱦ�Ϊ0.100mol?L-1HCl��CH3COOH�Ļ��Һ���絼��������ͼ��ʾ������˵����ȷ���ǣ�������
��2013?����һģ���絼�ʿ����ں����������Һ����������С���ҵ絼��Խ����Һ�ĵ�������Խǿ�������£���0.100mol?L-1��NH3?H2O �ζ�10.00mL Ũ�Ⱦ�Ϊ0.100mol?L-1HCl��CH3COOH�Ļ��Һ���絼��������ͼ��ʾ������˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �絼�ʿ����ں����������Һ����������С�������£���0.100mol/LNH3?H2O�ζ�10.00mLŨ�Ⱦ�Ϊ0.100mol/L HCl��CH3COOH�Ļ����Һ������������ͼ��ʾ������˵����ȷ���ǣ�������
�絼�ʿ����ں����������Һ����������С�������£���0.100mol/LNH3?H2O�ζ�10.00mLŨ�Ⱦ�Ϊ0.100mol/L HCl��CH3COOH�Ļ����Һ������������ͼ��ʾ������˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�絼�ʿ����ں����������Һ����������С�������£���0.100�ζ� 10.00 mL Ũ�Ⱦ�Ϊ 0.100 mol/L HCl��CH3COOH�Ļ����Һ��������������ͼ��ʾ������˵����ȷ���ǣ�)
A. �ٵ���Һ��C(H+)Ϊ
B. ��Һ�¶ȸߵ�Ϊ�١��ۡ���
C. �۵���Һ����
D. �۵����������Ŀ����ʹ��Һ�絼���Խ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ����ʮУ�������ʽ������������ۺ��Ծ���ѧ���֣������棩 ���ͣ�ѡ����
�絼�ʿ����ں����������Һ����������С�������£���0.100 �ζ� 10.00 mL Ũ�Ⱦ�Ϊ 0.100 mol/L HCl��CH3COOH�Ļ����Һ��������������ͼ��ʾ������˵����ȷ���ǣ�)
�ζ� 10.00 mL Ũ�Ⱦ�Ϊ 0.100 mol/L HCl��CH3COOH�Ļ����Һ��������������ͼ��ʾ������˵����ȷ���ǣ�)
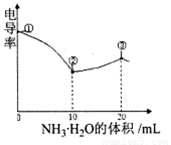
A. �ٵ���Һ��C(H+)Ϊ
B. ��Һ�¶ȸߵ�Ϊ�١��ۡ���
C. �۵���Һ����
D. �۵����������Ŀ����ʹ��Һ�絼���Խ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������������и������ϣ����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
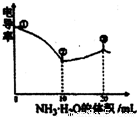
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com