
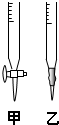
 ij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4?xH2O����x��ֵ��ͨ���������ϸ�С��ͬѧ��֪������������ˮ����ˮ��Һ����������KMnO4��Һ������Ӧ2MnO4-+5H2C2O4+6H+�T2Mn2++10CO2��+8H2O������ͬѧ���ø÷�Ӧԭ������˵ζ��ķ����ⶨxֵ��
ij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4?xH2O����x��ֵ��ͨ���������ϸ�С��ͬѧ��֪������������ˮ����ˮ��Һ����������KMnO4��Һ������Ӧ2MnO4-+5H2C2O4+6H+�T2Mn2++10CO2��+8H2O������ͬѧ���ø÷�Ӧԭ������˵ζ��ķ����ⶨxֵ��| �ζ����� | ���������Һ�����mL�� | 0.1000mol/LKMnO4����Һ�����mL�� | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| ��һ�� | 25.00 | 0.00 | 10.02 |
| �ڶ��� | 25.00 | 0.22 | 11.32 |
| ������ | 25.00 | 1.56 | 11.54 |


 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
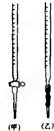 �Ҷ����������ᣬij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4?xH2O����xֵ��ͨ���������ϸ�С��ͬѧͨ�������ѯ�ã�����������ˮ��ˮ��Һ����������KMnO4��Һ���еζ���
�Ҷ����������ᣬij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4?xH2O����xֵ��ͨ���������ϸ�С��ͬѧͨ�������ѯ�ã�����������ˮ��ˮ��Һ����������KMnO4��Һ���еζ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| 11a |
| 36 |
| 11a |
| 20 |
| 11a |
| 36 |
| 11a |
| 20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
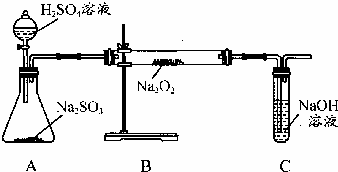 ij��ѧѧϰС���ͬѧΪ̽�������������������ķ�Ӧ������ͼ��ʾ��װ�ý���ʵ�飮ͨ����������������ǵ�ľ�������Թ�C��ľ����ȼ����ش���������
ij��ѧѧϰС���ͬѧΪ̽�������������������ķ�Ӧ������ͼ��ʾ��װ�ý���ʵ�飮ͨ����������������ǵ�ľ�������Թ�C��ľ����ȼ����ش����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
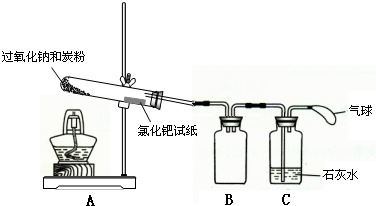 �ƵĻ����ﶼ�й㷺��Ӧ�ã�����Na2O2����һ�ֳ�������������ij��ѧѧϰС���ͬѧ��ͨ������ʵ��̽��̿����Na2O2��Ӧ�IJ��
�ƵĻ����ﶼ�й㷺��Ӧ�ã�����Na2O2����һ�ֳ�������������ij��ѧѧϰС���ͬѧ��ͨ������ʵ��̽��̿����Na2O2��Ӧ�IJ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com