


 ���ݼ���ϵ�д�
���ݼ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 1 |
| 2 |
| 3 |
| 2 |

| c4(H2O) |
| c4(H2) |
| c4(H2O) |
| c4(H2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �ŵ� | ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ�Ͳ�һ��2012�������ѧ����ĩ��⻯ѧ���� ���ͣ�022
(1)��֪��
Fe(s)��1/2O2(g)��FeO(s)����H����272.0 kJ��mol��1
2Al(s)��3/2O2(g)��Al2O3(s)����H����1675.7 kJ��mol��1
Al��FeO�������ȷ�Ӧ���Ȼ�ѧ����ʽ��________��
(2)��Ӧ�����������Ϊ��̬��ij���淴Ӧ�ڲ�ͬ�����µķ�Ӧ���̷ֱ�ΪA��B������ͼ��ʾ��
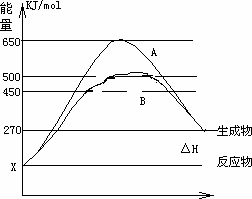
��
��ͼ�жϸ÷�Ӧ��________(������š�)�ȷ�Ӧ������Ӧ�ﵽƽ��������������䣬�����¶ȣ���Ӧ���ת����________(���������С�����䡱)��
����B���̱����˷�Ӧ���õ�����Ϊ________(ѡ�����������ĸ)A�������¶�
B������Ӧ���Ũ��
C�������¶�
D��ʹ���˴���
(3)1000��
ʱ���������������������з�Ӧ��Na2SO4(s)��4H2(g)�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ________����֪K1000����K1200������÷�Ӧ��________��Ӧ(����ȡ����ȡ�)��
�����й����ӷ���ʽ˵��������Ӧ���ù�������ˮ��Һ�������________
(4)������
�����ȡ0.1 mol��L��1HA��Һ��0.1 mol��L��1��NaOH��Һ��������(��Ϻ���Һ����ı仯���Բ���)����û��Һ��pH��8����ش�������������
�����Һ��ˮ�������c(H+)��0.1 mol��L��1��NaOH��Һ��ˮ�������c(H+)�Ƚ�________(������������)����
��֪NH4A��ҺΪ��������֪��HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶ�(NH4)2CO3��Һ��pH________7(������������)����ͬ�¶����������ʵ���Ũ�ȵ���������Һ��pH�ɴ�С������˳��Ϊ________��(�����)a��NH4HCO3
b��NH4A
c��(NH4)2CO3
d��NH4Cl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�߰���ѧ2011��2012ѧ��߶���ѧ�����п��Ի�ѧ���� ���ͣ�058
��Ҫ��ش���������
(1)�ñ�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�÷�̪��ָʾ�������в����лᵼ��ʵ����ƫ�͵���________(�����)
�ټ�ʽ�ζ���������ˮϴ����û���ñ�Һ��ϴ
������ʽ�ζ��ܼӴ���Һʱ����������ˮϴ����ĵζ���δ�ô���Һ��ϴ
����ƿ������ˮϴ����û���ô���Һ��ϴ
�ܵζ�ǰ�ζ��ܼ�������ݣ��ζ���������ʧ
���յ����ʱ���ӣ���������������ȷ
(2)ij����С��ͬѧ����ͼ��װ�ý���ʵ�飬�Դ��������⣺
������ʼʱ����K��a���ӣ����������绯ѧ��ʴ�е�________��ʴ��
������ʼʱ����K��b���ӣ����ܷ�Ӧ�����ӷ���ʽΪ________��
(3)��֪��Ǧ�����ܵĻ�ѧ����ʽΪ��Pb��PbO2��2H2SO4![]() 2PbSO4��2H2O
2PbSO4��2H2O
��Ǧ�����ڷŵ�ʱ������ӦΪ________��
��Ǧ�����ڳ��ʱ������ӦΪ________��
�����Ǧ�����ڷŵ�ʱ��·����2 mol����ת��ʱ������H2SO4________mol��
(4)�����£����ȡ0.1 mol/L��HA��Һ��0.1 mol/L��NaOH��Һ��������(���Ի�Ϻ���Һ����ı仯)����û����Һ��pH��8��������Һ��������ʽ�ľ�ȷ������(���������)��c(OH��)��c(HA)��________mol/L��
(5)��Cl����Al3+��HSO4����K+��HS�����������У�ֻ��ˮ�ⲻ�ܵ����������________��ֻ�ܵ��벻��ˮ���������________�����ܵ�������ˮ���������________��д����ˮ�����ӵ�ˮ�����ӷ���ʽ________��________��
(6)��֪25��ʱ��Mg(OH)2���ܶȻ�����Ksp��5.6��10��12�����ij��Һ��pH��13������¶�����Һ�е�c(Mg2+)��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ���������ѧУ2012�����9��������⻯ѧ���� ���ͣ�022
������
�����ȡ0.1 mol��L��1��HA��Һ��0.1 mol��L��1��NaOH��Һ��������(��Ϻ���Һ����ı仯���Բ���)����û��Һ��pH��8����ش�������������
�����Һ��ˮ�������c(H+)��0.1 mol��L��1��NaOH��Һ��ˮ�������c(H+)�Ƚ�________(������������)����
��֪NH4A��ҺΪ��������֪��HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶ�(NH4)2CO3��Һ��pH________7(������������)����ͬ�¶����������ʵ���Ũ�ȵ�������������Һ��pH�ɴ�С������˳��Ϊ________��(�����)a��NH4HCO3
b��NH4A
c��(NH4)2CO3
d��NH4Cl
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com