

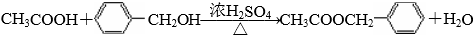 ��
�� ��
�� CH2CH2OOCH��
CH2CH2OOCH�� COOCH2CH3��
COOCH2CH3�� CH2COOCH3��
CH2COOCH3�� 00CCH2CH3��
00CCH2CH3�� CH��CH3��OOCH��
CH��CH3��OOCH�� ���� ��ϩ��ˮ�ڴ��������·����ӳɷ�Ӧ�����Ҵ�����A���Ҵ����Ҵ������������������ᣬ��B������ױ��ڹ�����������������������ȡ����Ӧ����CΪ ��C���������Ƶ�ˮ��Һ�з�������ȡ����Ӧ����ôDӦΪ
��C���������Ƶ�ˮ��Һ�з�������ȡ����Ӧ����ôDӦΪ ����D�ܱ�����ΪE���ҽ�ϸ�������Ϣ��RCHO+CH3COOR��$\stackrel{CH_{3}CH_{2}ONa}{��}$RCH=CHCOOR�䣬��ôӦEΪ
����D�ܱ�����ΪE���ҽ�ϸ�������Ϣ��RCHO+CH3COOR��$\stackrel{CH_{3}CH_{2}ONa}{��}$RCH=CHCOOR�䣬��ôӦEΪ ���ݴ��ƶϵó�FΪ��
���ݴ��ƶϵó�FΪ�� ����ôXΪ
����ôXΪ ���ݴ˽�ϸ�С��شɣ�
���ݴ˽�ϸ�С��شɣ�
��� �⣺��ϩ��ˮ�ڴ��������·����ӳɷ�Ӧ�����Ҵ�����A���Ҵ����Ҵ������������������ᣬ��B������ױ��ڹ�����������������������ȡ����Ӧ����CΪ ��C���������Ƶ�ˮ��Һ�з�������ȡ����Ӧ����ôDӦΪ
��C���������Ƶ�ˮ��Һ�з�������ȡ����Ӧ����ôDӦΪ ����D�ܱ�����ΪE���ҽ�ϸ�������Ϣ��RCHO+CH3COOR��$\stackrel{CH_{3}CH_{2}ONa}{��}$RCH=CHCOOR�䣬��ôӦEΪ
����D�ܱ�����ΪE���ҽ�ϸ�������Ϣ��RCHO+CH3COOR��$\stackrel{CH_{3}CH_{2}ONa}{��}$RCH=CHCOOR�䣬��ôӦEΪ ���ݴ��ƶϵó�FΪ��
���ݴ��ƶϵó�FΪ�� ����ôXΪ
����ôXΪ ��
��
��1��A���Ҵ���A�й����ŵ��������ǻ���C��D�ķ�Ӧ����Ϊȡ����Ӧ��
�ʴ�Ϊ���ǻ���ȡ����Ӧ��
��2��BΪ���ᣬDΪ���״������߷���������Ӧ�������ᱽ��������ѧ��Ӧ����ʽΪ��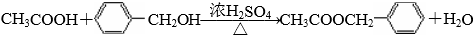 ��
��
�ʴ�Ϊ��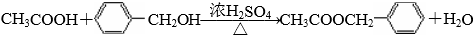 ��
��
��3����������ķ�����֪��EΪ ��
��
�ʴ�Ϊ�� ��
��
��4��A��X�к����������ܷ���ˮ�ⷴӦ����A��ȷ��
B��X�в�����ȩ�������ܷ���������Ӧ����B����
C��X�к��б���������Ũ���ᷢ��ȡ����Ӧ����C����
D��X�к���̼̼˫������ʹBr2/CCl4��Һ��ɫ����D��ȷ��
��ѡAD��
��5��F�ж���ͬ���칹�壬����������һȡ�����ṹ��ͬ���칹��������������ĸ��Ľṹ��ʽ�ǣ� CH2CH2OOCH��
CH2CH2OOCH�� COOCH2CH3��
COOCH2CH3�� CH2COOCH3��
CH2COOCH3�� 00CCH2CH3��
00CCH2CH3�� CH��CH3��OOCH��
CH��CH3��OOCH��
�ʴ�Ϊ�� CH��CH3��OOCH��
CH��CH3��OOCH��
���� ������Ҫ��������л���ĺϳ����л�����ƶϣ�������ճ����л�������������Լ�ץס������Ϣ�����ǹؼ����Ѷ��еȣ�ע���л������ŵ����ʵ�������ã�


 һ����������ϵ�д�
һ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��FeBr2��Һ��ͨ������Cl2��Cl2+2Br-�T2Cl-+Br2 | |
| B�� | ��ȥMgCl2��Һ��������FeCl3��3Mg+2Fe3+�T2Fe+3Mg2+ | |
| C�� | ���а�ˮ��BaCl2��Һ��ͨ��SO2��SO2+2NH3•H2O+Ba2+�TBaSO3��+2NH4++H2O | |
| D�� | ������������̼ͨ���Ȼ�����Һ�У�Ca2++CO2+H2O�TCaCO3+2H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��ҩʹ�� ��ҩʹ�� | B�� |  ��ʳ��� | C�� |  ת������ | D�� |  ����ұ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȡ��̼���ڼ�����ʽ | |
| B�� | ���չ涨�������������з������ | |
| C�� | �������մ����ո� | |
| D�� | ����ũ���������������֪ʶ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ϩ�����к�̼̼˫�� | B�� | ��֬��������Ӧ���ڼӳɷ�Ӧ | ||
| C�� | ������3��ͬ���칹�� | D�� | �Ե���Ϊԭ�Ͽ����Ʊ��Ҵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cr2O72- | B�� | Cl2 | C�� | MnO4- | D�� | H2O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ��һ�����л�ѧ�Ծ��������棩 ���ͣ������
(1)����ÿ��ֱ�����������������п������һ�����ʵ�����ʽ������(����)___________
�� | �� | �� | �� | �� |
�������������� | ������� | ��Һ�������ٷֱ�Ũ�� | ��״���������Ħ����� | �DZ�״����ij���ʵ����� |
�����ӵ����� | �����ܶ� | ��Һ��� | ��״������������ | ���ʵ�Ħ������ |
(2)�ڱ�״���£���6.72LCH4�� ��3.01��1023��HCl���ӣ� ��13.6gH2S�� ��0.2molNH3�����ж������������������ȷ����(��д����)___________
A��������>��>��>�� B���ܶȢ�>��>��>�� C�������>��>��>�� D����ԭ������>��>��>��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com