
| 1.8X 10-10 |
| 1.8X 10-16 |
| 3 | 1.8X 10-12 |
| 1.8X 10-10 |
| 1.8X 10-16 |
| 3 | 1.8X 10-12 |


 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | �����Լ� | ʵ������ |
| ��һ����Һ | �μ������ĵ���KI��Һ | ����ɫ |
| �ڶ�����Һ | �μ��������λ���BaCl2��Һ | �а�ɫ���� |
| ��������Һ | �μ���Һ�����ȣ����������������Һ�����V�������ɵij����������������ϵ��n������ͼ�� |  |
| ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
| c��NO2��/mol?L-1 | 1.00��10-3 | 4.50��10-4 | 2.50��10-4 | 1.50��10-4 | 1.00��10-4 | 1.00��10-4 |
| c��SO2��/mol?L-1 | 3.60��10-3 | 3.05��10-3 | 2.85��10-3 | 2.75��10-3 | 2.70��10-3 | 2.70��10-3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
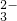 S0
S0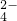 ��N03-��N02-�ȣ�Ϊȷ������ɣ��ֱ��������4��ʵ�飬������ȷ����Ʒ�в���S0
��N03-��N02-�ȣ�Ϊȷ������ɣ��ֱ��������4��ʵ�飬������ȷ����Ʒ�в���S0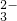 ��ʵ�������______��������ʵ�������ţ�������ʵ����������Ϊ�Ƿ���N02-����������С����������С�������ԭ����______��
��ʵ�������______��������ʵ�������ţ�������ʵ����������Ϊ�Ƿ���N02-����������С����������С�������ԭ����______��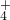 ��Cl-�������ӣ�����c��NH
��Cl-�������ӣ�����c��NH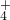 ����c��Cl-�������������ʵĻ�ѧʽ�ֱ���______��
����c��Cl-�������������ʵĻ�ѧʽ�ֱ���______���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㽭ʡģ���� ���ͣ������
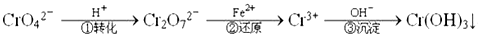
 Cr2O72��(��ɫ)+H2O
Cr2O72��(��ɫ)+H2O  Cr3+(aq)+3OH-(aq) �����£�Cr(OH)3���ܶȻ�Ksp=10-32��Ҫʹc(Cr3+)����10-5mol/L����Һ��pHӦ����_____��
Cr3+(aq)+3OH-(aq) �����£�Cr(OH)3���ܶȻ�Ksp=10-32��Ҫʹc(Cr3+)����10-5mol/L����Һ��pHӦ����_____�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�����и������ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
 S0
S0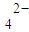 ��N03-��N02-�ȣ�Ϊȷ������ɣ��ֱ��������4��ʵ�飬������ȷ����Ʒ�в���S0
��N03-��N02-�ȣ�Ϊȷ������ɣ��ֱ��������4��ʵ�飬������ȷ����Ʒ�в���S0 ��ʵ������� ��������ʵ�������ţ�������ʵ����������Ϊ�Ƿ���N02-����������С����������С�������ԭ���� ��
��ʵ������� ��������ʵ�������ţ�������ʵ����������Ϊ�Ƿ���N02-����������С����������С�������ԭ���� �� ��Cl-�������ӣ�����c��NH
��Cl-�������ӣ�����c��NH ����c��Cl-�������������ʵĻ�ѧʽ�ֱ��� ��
����c��Cl-�������������ʵĻ�ѧʽ�ֱ��� ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com