

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
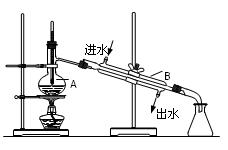
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
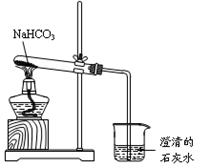
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
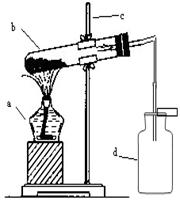
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 00mL0.2000 mol��L-1Na2CO3��Һ����ƿ��,��2��3�μ�����ָʾ������δ֪Ũ�ȵ��������ζ�0.2000 mol��L-1Na2CO3��Һ������ �жϵζ��յ�ﵽ��
00mL0.2000 mol��L-1Na2CO3��Һ����ƿ��,��2��3�μ�����ָʾ������δ֪Ũ�ȵ��������ζ�0.2000 mol��L-1Na2CO3��Һ������ �жϵζ��յ�ﵽ�� �����ߵ�ƽ��ֵΪ3.41�棬��ʵ�����к��ȡ�H= (��Ϻ���Һ�ı�����C = 4.18J����-1��g-1)��ʵ�����к��ȱ����� (ƫ�ߣ���ȣ�ƫ��)
�����ߵ�ƽ��ֵΪ3.41�棬��ʵ�����к��ȡ�H= (��Ϻ���Һ�ı�����C = 4.18J����-1��g-1)��ʵ�����к��ȱ����� (ƫ�ߣ���ȣ�ƫ��)�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ���ʣ����ʣ� | ���ӷ��� |
| A | CO2��HCl�� | ������ͨ��ʢ�б���NaHCO3��Һ��ϴ��ƿ |
| B | Cl2��HCl�� | ������ͨ��ʢ��NaOH��Һ��ϴ��ƿ |
| C | CaCO3��SiO2�� | ���������������ˡ�ϴ�� |
| D | Mg��Al�� | ��������NaOH��Һ����ˡ�ϴ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com