
| |||||||||||||||||||||||||||||||
(1) |
(2��) |
(2) |
6,5,4,3,2(1��),�ձ�(1��) |
(3) |
������ϡ��Ũ���ᣬ�����������ԣ����ٸ���Ʒ������ɣ�(1��); �������ܽ�HBr����ֹHBr�����ݳ���Ӱ���Ʒ�����ɣ�(1��) |
(4) |
Br2(1��),NaOH��Һ(1��)����Һȡ�²�(1��) |
(5) |
ȡ��Һ��IJ�Ʒ��������NaOH��Һ����(1��)���ڼ��Ⱥ����Һ�м�HNO3ʹ�������(1��)���������еμ�AgNO3(1��)�����г������ɣ�֤��C2H5Br���ɣ�(1��) |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| �� |
| �ܶ�/g?mL-1 | �е�/�� | �ܽ��� | |
| ������ | 1.461 | 38 | ������ˮ |
| �Ҵ� | 0.789 | 78 | ������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�� ������500mL0.2mol/LNa2CO3��Һ���ش��������⣺
��1��Ӧ��ȡ g Na2CO3��10H2O��
��2�����Ƹ���Һ���õ������� ____ ��
��3�����в���ʹ������ҺŨ��ƫ�͵��ǣ� ��
A������ƿδ���T����������Һ B��������ƿת����Һʱ������Һ�彦��
C��δϴ���ܽ������ձ� D������ʱ�����ӿ̶���
������ij��ѧС����ʵ��������ͼ�ṩ�������Ʊ������顣

��֪��
�ٷ�Ӧԭ����NaBr+H2SO4����Ũ����=== NaHSO4+HBr
HBr+C2H5OHC2H5Br+H2O
�ڷ�Ӧ��������NaBr(S)25g����ˮ�Ҵ�15mL��ŨH2SO4 30mL��ˮ15mL
����������Ҵ��IJ��������������±�
| �ܶ�/g��mL-1 | �е�/�� | �ܽ��� | |
| ������ | 1.461 | 38 | ������ˮ |
| �Ҵ� | 0.789 | 78 | ������ˮ |
�ش��������⣺
��4����������������˳���ǣ�1��( )��( )��( 4 )��( 3 )��( )�������֣�������Ϊ��Ӧ���������� �����ƿ�����ձ�������
��5������Ũ�������ǿ�����ԣ���Ӧ���������ʹ���������鳣�ʻ�ɫ���ø������� ��д��ѧʽ������ȥ�����ʵ��Լ��ͷ����� ��Ϊ�˼��ٸø���������ɣ����ݼ���ķ�Ӧ���ʵ���ȡ�� _________________________________________________________�Ĵ�ʩ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�� ������500mL0.2mol/LNa2CO3��Һ���ش��������⣺
��1��Ӧ��ȡ g Na2CO3��10H2O��
��2�����Ƹ���Һ���õ������� ____ ��
��3�����в���ʹ������ҺŨ��ƫ�͵��ǣ� ��
A������ƿδ���T����������Һ B��������ƿת����Һʱ������Һ�彦��
C��δϴ���ܽ������ձ� D������ʱ�����ӿ̶���
������ij��ѧС����ʵ��������ͼ�ṩ�������Ʊ������顣
��֪��
�ٷ�Ӧԭ����NaBr+H2SO4����Ũ����=== NaHSO4+HBr
HBr+C2H5OHC2H5Br+H2O
�ڷ�Ӧ��������NaBr(S)25g����ˮ�Ҵ�15mL��ŨH2SO4 30mL��ˮ15mL
����������Ҵ��IJ��������������±�
|
| �ܶ�/g��mL-1 | �е�/�� | �ܽ��� |
| ������ | 1.461 | 38 | ������ˮ |
| �Ҵ� | 0.789 | 78 | ������ˮ |
�ش��������⣺
��4����������������˳���ǣ�1��( )��( )��(4 )��( 3 )��( )�������֣�������Ϊ��Ӧ���������� �����ƿ�����ձ�������
��5������Ũ�������ǿ�����ԣ���Ӧ���������ʹ���������鳣�ʻ�ɫ���ø������� ��д��ѧʽ������ȥ�����ʵ��Լ��ͷ����� ��Ϊ�˼��ٸø���������ɣ����ݼ���ķ�Ӧ���ʵ���ȡ�� _________________________________________________________�Ĵ�ʩ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡִ����ѧ������ѧ�����п��Ի�ѧ�� ���ͣ������
��һ�� ������500mL0.2mol/LNa2CO3��Һ���ش��������⣺
��1��Ӧ��ȡ g Na2CO3��10H2O��
��2�����Ƹ���Һ���õ������� ____ ��
��3�����в���ʹ������ҺŨ��ƫ�͵��ǣ� ��
| A������ƿδ���T����������Һ | B��������ƿת����Һʱ������Һ�彦�� |
| C��δϴ���ܽ������ձ� | D������ʱ�����ӿ̶��� |

| | �ܶ�/g��mL-1 | �е�/�� | �ܽ��� |
| ������ | 1.461 | 38 | ������ˮ |
| �Ҵ� | 0.789 | 78 | ������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ������ѧ�����п��Ի�ѧ�� ���ͣ������
��һ�� ������500mL0.2mol/LNa2CO3��Һ���ش��������⣺
��1��Ӧ��ȡ g Na2CO3��10H2O��
��2�����Ƹ���Һ���õ������� ____ ��
��3�����в���ʹ������ҺŨ��ƫ�͵��ǣ� ��
A������ƿδ���T����������Һ B��������ƿת����Һʱ������Һ�彦��
C��δϴ���ܽ������ձ� D������ʱ�����ӿ̶���
������ij��ѧС����ʵ��������ͼ�ṩ�������Ʊ������顣
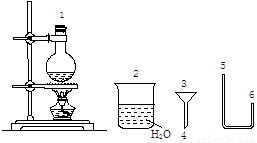
��֪��
�ٷ�Ӧԭ����NaBr+H2SO4����Ũ����=== NaHSO4+HBr
HBr+C2H5OHC2H5Br+H2O
�ڷ�Ӧ��������NaBr(S)25g����ˮ�Ҵ�15mL��ŨH2SO4 30mL��ˮ15mL
����������Ҵ��IJ��������������±�
|
|
�ܶ�/g��mL-1 |
�е�/�� |
�ܽ��� |
|
������ |
1.461 |
38 |
������ˮ |
|
�Ҵ� |
0.789 |
78 |
������ˮ |
�ش��������⣺
��4����������������˳���ǣ�1��( )��( )��( 4 )��( 3 )��( )�������֣�������Ϊ��Ӧ���������� �����ƿ�����ձ�������
��5������Ũ�������ǿ�����ԣ���Ӧ���������ʹ���������鳣�ʻ�ɫ���ø������� ��д��ѧʽ������ȥ�����ʵ��Լ��ͷ����� ��Ϊ�˼��ٸø���������ɣ����ݼ���ķ�Ӧ���ʵ���ȡ�� _________________________________________________________�Ĵ�ʩ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com