
 �����ʣ�����ҽҩ����ˮ���Ȼ��ƣ�
�����ʣ�����ҽҩ����ˮ���Ȼ��ƣ�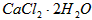 ����������Ϊ97��0%~103��0%������Ҫ��������
����������Ϊ97��0%~103��0%������Ҫ��������
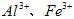 ������
������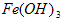 �Ƿ������ȫ�����������___________________��
�Ƿ������ȫ�����������___________________��  ת��Ϊ
ת��Ϊ ��
�� 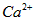 ������ʱˮ�⣻
������ʱˮ�⣻ �����ķ����ǣ�
�����ķ����ǣ� ��Һ�ζ����յ㣬����
��Һ�ζ����յ㣬���� ��Һ�����ƽ��ֵΪ20��39mL��
��Һ�����ƽ��ֵΪ20��39mL��  ����������Ϊ___________________��
����������Ϊ___________________��  ����������ƫ�ߣ��ⶨ�����в��������ɺ��ԣ��������ԭ����__________________��___________________��
����������ƫ�ߣ��ⶨ�����в��������ɺ��ԣ��������ԭ����__________________��___________________�� 
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
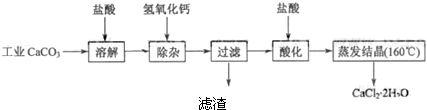
��10�֣�ҽ���Ȼ��ƿ������������ơ�������������ҩ��Թ�ҵ̼��ƣ���������![]() ����־������ҽҩ����ˮ���Ȼ��ƣ�
����־������ҽҩ����ˮ���Ȼ��ƣ�![]() ����������Ϊ97.0%~103.0%������Ҫ�������£�
����������Ϊ97.0%~103.0%������Ҫ�������£�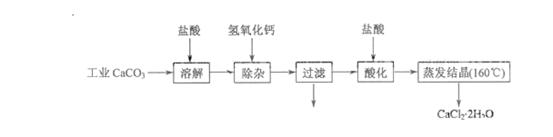
��1�����Ӳ����Ǽ����������ƣ�������Һ��![]() Ϊ8.0~8.5���Գ�ȥ��Һ�е�����
Ϊ8.0~8.5���Գ�ȥ��Һ�е�����![]() ������
������![]() �Ƿ������ȫ����������� �� ��
�Ƿ������ȫ����������� �� ��
��2���ữ�����Ǽ������ᣬ������Һ��![]() ԼΪ4.0����Ŀ���У��ٽ���Һ�е�����
ԼΪ4.0����Ŀ���У��ٽ���Һ�е�����![]() �� �ڷ�ֹ
�� �ڷ�ֹ![]() ������ʱˮ�⣻�� �� ��
������ʱˮ�⣻�� �� ��
��3���ⶨ��Ʒ��![]() �����ķ����ǣ�a.��ȡ0.7500g��Ʒ���ܽ⣬��250mL����ƿ�ж��ݣ�b.��ȡ25.00mL������Һ����ƿ�У�c.��0.05000
�����ķ����ǣ�a.��ȡ0.7500g��Ʒ���ܽ⣬��250mL����ƿ�ж��ݣ�b.��ȡ25.00mL������Һ����ƿ�У�c.��0.05000![]()
![]() ��Һ�ζ����յ㣬����
��Һ�ζ����յ㣬����![]() ��Һ�����ƽ��ֵΪ20.39mL��
��Һ�����ƽ��ֵΪ20.39mL��
�������ⶨ��������Ҫ��Һ��ϴ�������� �� ��
�ڼ���������Ʒ��![]() ����������Ϊ �� ��
����������Ϊ �� ��
�����������취�ⶨ����Ʒ��![]() ����������ƫ�ߣ��ⶨ�����в��������ɺ��ԣ��������ԭ���� �� �� �� ��
����������ƫ�ߣ��ⶨ�����в��������ɺ��ԣ��������ԭ���� �� �� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ҽ���Ȼ��ƿ������������ơ�������������ҩ��Թ�ҵ̼��ƣ���������Na+��Al3+��Fe3+�����ʣ�����ҽҩ����ˮ���Ȼ��ƣ�CaCl2��2H2O����������Ϊ97.0%~103.0%������Ҫ�������£�

��1�����Ӳ����Ǽ���Ca(OH)2��������ҺpHΪ8.0~8.5���Գ�ȥ��Һ�е�����Al3+��Fe3+������Fe(OH)3�Ƿ������ȫ��ʵ������� ��
��2���ữ�����Ǽ������ᣬ������Һ��pHԼΪ4.0����Ŀ���У��ٷ�ֹCa2+������ʱˮ�⣻�� ��
��3![]() ���ⶨ��Ʒ��Cl-�����ķ����ǣ�a.��ȡ0.7500 g��Ʒ���ܽ⣬��250 mL����ƿ�ж��ݣ�b.��ȡ25.00 mL������Һ����ƿ�У�c.��0.05000 mol��L-1 AgNO3��Һ�ζ����յ㣬����AgNO3��Һ�����ƽ��ֵΪ20.39 mL��
���ⶨ��Ʒ��Cl-�����ķ����ǣ�a.��ȡ0.7500 g��Ʒ���ܽ⣬��250 mL����ƿ�ж��ݣ�b.��ȡ25.00 mL������Һ����ƿ�У�c.��0.05000 mol��L-1 AgNO3��Һ�ζ����յ㣬����AgNO3��Һ�����ƽ��ֵΪ20.39 mL��
�������ⶨ������������Һ��ϴ�������� �� �ڼ���������Ʒ��CaCl2��2H2O����������Ϊ ��
���������������ⶨ����Ʒ��CaCl2��2H2O����������ƫ�ߣ��ⶨ�����в��������ɺ��ԣ��������ԭ���� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ӱ�ʡ������ѧ������ѧ�ڵ����ο��Ի�ѧ�Ծ� ���ͣ������
ҽ���Ȼ��ƿ������������ơ�������������ҩ��Թ�ҵ̼��ƣ���������Na+��Al3+��Fe3+�����ʣ�����ҽҩ����ˮ���Ȼ��ƣ�CaCl2��2H2O����������Ϊ97.0%~103.0%������Ҫ�������£�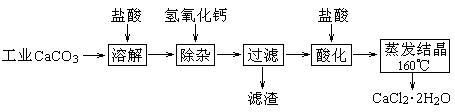
��1�����Ӳ����Ǽ���Ca(OH)2��������ҺpHΪ8.0~8.5���Գ�ȥ��Һ�е�����Al3+��Fe3+������Fe(OH)3�Ƿ������ȫ��ʵ������� ��
��2���ữ�����Ǽ������ᣬ������Һ��pHԼΪ4.0����Ŀ���У��ٷ�ֹCa2+������ʱˮ�⣻�� ��
��3 ���ⶨ��Ʒ��Cl-�����ķ����ǣ�a.��ȡ0.7500 g��Ʒ���ܽ⣬��250 mL����ƿ�ж��ݣ�b.��ȡ25.00 mL������Һ����ƿ�У�c.��0.05000 mol��L-1 AgNO3��Һ�ζ����յ㣬����AgNO3��Һ�����ƽ��ֵΪ20.39 mL��
���ⶨ��Ʒ��Cl-�����ķ����ǣ�a.��ȡ0.7500 g��Ʒ���ܽ⣬��250 mL����ƿ�ж��ݣ�b.��ȡ25.00 mL������Һ����ƿ�У�c.��0.05000 mol��L-1 AgNO3��Һ�ζ����յ㣬����AgNO3��Һ�����ƽ��ֵΪ20.39 mL��
�������ⶨ������������Һ��ϴ�������� �� �ڼ���������Ʒ��CaCl2��2H2O����������Ϊ ��
���������������ⶨ����Ʒ��CaCl2��2H2O����������ƫ�ߣ��ⶨ�����в��������ɺ��ԣ��������ԭ���� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ӱ�ʡ������ѧ�ڵ����ο��Ի�ѧ�Ծ� ���ͣ������
ҽ���Ȼ��ƿ������������ơ�������������ҩ��Թ�ҵ̼��ƣ���������Na+��Al3+��Fe3+�����ʣ�����ҽҩ����ˮ���Ȼ��ƣ�CaCl2��2H2O����������Ϊ97.0%~103.0%������Ҫ�������£�
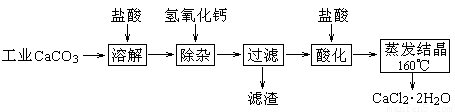
��1�����Ӳ����Ǽ���Ca(OH)2��������ҺpHΪ8.0~8.5���Գ�ȥ��Һ�е�����Al3+��Fe3+������Fe(OH)3�Ƿ������ȫ��ʵ������� ��
��2���ữ�����Ǽ������ᣬ������Һ��pHԼΪ4.0����Ŀ���У��ٷ�ֹCa2+������ʱˮ�⣻�� ��
��3���ⶨ��Ʒ��Cl-�����ķ����ǣ�a.��ȡ0.7500 g��Ʒ���ܽ⣬��250 mL����ƿ�ж��ݣ�b.��ȡ25.00 mL������Һ����ƿ�У�c.��0.05000 mol��L-1 AgNO3��Һ�ζ����յ㣬����AgNO3��Һ�����ƽ��ֵΪ20.39 mL��
�������ⶨ������������Һ��ϴ�������� �� �ڼ���������Ʒ��CaCl2��2H2O����������Ϊ ��
���������������ⶨ����Ʒ��CaCl2��2H2O����������ƫ�ߣ��ⶨ�����в��������ɺ��ԣ��������ԭ���� �� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com