

 $\stackrel{��}{��}$
$\stackrel{��}{��}$
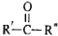 ��
��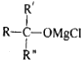 $\stackrel{H_{2}O}{��}$
$\stackrel{H_{2}O}{��}$


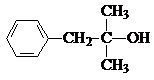


���� ����ϢI��Y�Ľṹ�����ƿ�֪GΪ
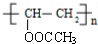 ����N�ķ���ʽ��֪��������M����������Ӧ����N����M�ķ���ʽΪ��C12H16O2+H2O-C2H4O2=C10H14O����N�ķ����к���3��������M�к���2�����������ϢII��֪��KΪ
����N�ķ���ʽ��֪��������M����������Ӧ����N����M�ķ���ʽΪ��C12H16O2+H2O-C2H4O2=C10H14O����N�ķ����к���3��������M�к���2�����������ϢII��֪��KΪ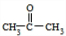 ��MΪ
��MΪ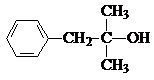
 ���ݴ˷������
���ݴ˷������
��� �⣺����ϢI��Y�Ľṹ�����ƿ�֪GΪ
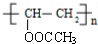 ����N�ķ���ʽ��֪��������M����������Ӧ����N����M�ķ���ʽΪ��C12H16O2+H2O-C2H4O2=C10H14O����N�ķ����к���3��������M�к���2�����������ϢII��֪��KΪ
����N�ķ���ʽ��֪��������M����������Ӧ����N����M�ķ���ʽΪ��C12H16O2+H2O-C2H4O2=C10H14O����N�ķ����к���3��������M�к���2�����������ϢII��֪��KΪ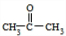 ��MΪ
��MΪ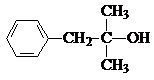
 ��
��
��1��������������֪��CΪCH3CHO����������ȩ���ʴ�Ϊ����ȩ��
��2����B��һ������������PVAc��֬�Ļ�ѧ����ʽ�� ��
��
�ʴ�Ϊ�� ��
��
��3��������������֪��D�Ľṹ��ʽ��CH3CH=CHCHO��
�ʴ�Ϊ��CH3CH=CHCHO��
��4��E��F�Ļ�ѧ����ʽΪ��2CH3CH2CH2CH2OH+O2$��_{��}^{Cu}$2CH3CH2CH2CHO+2H2O��
�ʴ�Ϊ��2CH3CH2CH2CH2OH+O2$��_{��}^{Cu}$2CH3CH2CH2CHO+2H2O��
��5��F��G�Ļ�ѧ����ʽΪ��2HCHO+CH3CH2CH2CHO$\stackrel{OH-}{��}$
�ʴ�Ϊ��2HCHO+CH3CH2CH2CHO$\stackrel{OH-}{��}$
��6��������������֪��M�Ľṹ��ʽ��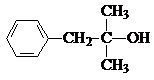
�ʴ�Ϊ��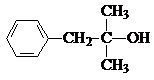
��7��ͨ�����Ϸ���֪��A����B�ķ�Ӧ�Ǽӳɷ�Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��
��8�������й�˵������ȷ���� ������ĸ��ţ���
a��NΪ �����������������������ʣ���a��ȷ��
�����������������������ʣ���a��ȷ��
b��CΪCH3CHO������ȩ��KΪ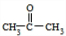 ������ͪ�����߲�����ͬϵ���b����
������ͪ�����߲�����ͬϵ���b����
c��PVAc��֬Ϊ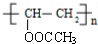 ������������һ�������£�������NaOH��Һ��Ӧ����c��ȷ��
������������һ�������£�������NaOH��Һ��Ӧ����c��ȷ��
d��������������֪����Ӧ�١��ڶ���������H2�ļӳɷ�Ӧ����d��ȷ��
��ѡ��acd��
��9���÷�Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��10����������������B��CH3COOCH=CH2����ͬ���칹�壺a����B����ͬ�Ĺ����ţ�����������̼̼˫����b����ʽ�ṹ������������ͬ���칹��Ϊ��
�ʴ�Ϊ��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ƶ�������֪ʶǨ������������ijЩ���ʽṹ��ʽ����Ӧ�����������Ϣ�����ƶϣ���ȷ�����ż������ʹ�ϵ�ǽⱾ��ؼ�����Ŀ�ѶȲ���


 ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��֪AΪ���ʣ�B��C��D��EΪ���������֮�������ͼת����ϵ��
��֪AΪ���ʣ�B��C��D��EΪ���������֮�������ͼת����ϵ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3-CH=CH2�� | B�� |  �� �� | C�� |  �� �� | D�� | ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����NH4HSO4���壬v��H2������ | B�� | ��������ˮ��v��H2����С | ||
| C�� | ����CH3COONa���壬v��H2����С | D�� | �μ�����CuSO4��Һ��v��H2���ӿ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ԭ�Ӱ뾶 | �縺�� | �۵� | �е� |
| Al��Si | N��O | ���ʯ������� | CH4��SiH4 |
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯ���ѻ��ɻ����ϩ����ϩ�Ͷ���ϩ | |
| B�� | ˮ���������������ϼ��ͷ���� | |
| C�� | �������ᴿ�����ʲ��������������� | |
| D�� | ��������������������Ʒ�Ļ���ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na��Mg��Alԭ�������������������� | |
| B�� | N��O��FԪ����������������� | |
| C�� | Na��K��Rb�縺����С | |
| D�� | P��S��ClԪ����ۺ���������������ǿ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com