
��1 mol��I2(g)��2 mol��H2����2 L�ܱ������У���һ���¶��·�����Ӧ��
I2(g)��H2(g)![]() 2HI(g)����H��0������ƽ�⣮HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ��
2HI(g)����H��0������ƽ�⣮HI���������w(HI)��ʱ��仯��ͼ����(��)��ʾ��
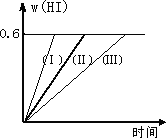
(1)��ƽ��ʱ��I2(g)�����ʵ���Ũ��Ϊ________��
(2)���ı䷴Ӧ�������ڼ�������w(HI)�ı仯������(��)��ʾ������������w(HI)�ı仯������(��)��ʾ���������������________����������������________(�����������������)
�ٺ��������£������¶ȣ�
�ں��������£������¶ȣ�
�ۺ��������£���С��Ӧ���������
�ܺ��������£�����Ӧ���������
�ݺ��º��������£������ʵ�������
(3)�������¶Ȳ��䣬����һ����ͬ��2 L�ܱ������м���a mol��I2(g)��b mol��H2(g)��c mol��HI(a��b��c������0)��������Ӧ����ƽ��ʱ��HI�����������Ϊ0.6����a��b��c�Ĺ�ϵ��________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ��������2011��2012ѧ��߶���ѧ�����п��Ի�ѧ���� ���ͣ�013
|
һ���¶��£���1 mol��I2(g)��2 mol��H2����2 L�ܱ������У�������Ӧ��I2(g)��H2(g) �ٺ��������£������¶� �ں��������£���С��Ӧ������� �ۺ��º��������£������ʵ����� �ܺ��º��������£��ټ���1 mol��I2(g)��2 mol��H2 �ݺ��������£�����Ӧ�������
| |
| [����] | |
A�� |
�٢� |
B�� |
�٢ڢ� |
C�� |
�ڢۢ� |
D�� |
�ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�ĵ���У2011��2012ѧ��߶���ѧ�ڵڶ����¿���ѧ���� ���ͣ�022
��1 mol��I2(g)��2 mol��H2����5 L�ܱ������У���һ���¶��·�����Ӧ��I2(g)��H2(g)![]() 2HI(g)����H��0�����ﵽƽ�⣮HI���������w(HI)��ʱ��仯������(��)��ʾ��
2HI(g)����H��0�����ﵽƽ�⣮HI���������w(HI)��ʱ��仯������(��)��ʾ��
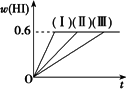
(1)�ﵽƽ��ʱ��I2(g)�����ʵ���Ũ��Ϊ________��
(2)���ı䷴Ӧ�������ڼ�������w(HI)�ı仯������(��)��ʾ������������w(HI)�ı仯������(��)��ʾ���������������________��������������________(�����������������)��
�ٺ��������£������¶�
�ں��������£������¶�
�ۺ��������£���С��Ӧ�������
�ܺ��������£�����Ӧ�������
�ݺ��º��������£������ʵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008����������һ�и����꼶�������¿����ԡ������ۺ��Ծ���ѧ���� ���ͣ�022
��1 mol��I2(g)��2 mol��H2(g)����ij2L�ܱ������У���һ���¶��·�����Ӧ��I2(g)��H2(g)![]() 2HI(g)����H��0�����ﵽƽ�⣮HI�����������(HI)��ʱ��仯��ͼ����(��)��ʾ��
2HI(g)����H��0�����ﵽƽ�⣮HI�����������(HI)��ʱ��仯��ͼ����(��)��ʾ��
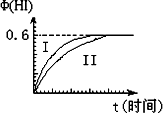
(1)��ƽ��ʱ��I2(g)�����ʵ���Ũ��Ϊ________mol��L��1��
(2)���ı䷴Ӧ��������ij��������(HI)�ı仯������
(��)��ʾ���������������________(�����������������)��
�ٺ��������£������¶�
�ں��������£������¶�
�ۺ��������£���С��Ӧ�������
�ܺ��������£�����Ӧ�������
�ݺ��¡����������£������ʵ�����
(3)�������¶Ȳ��䣬����һ��ͬ��2 L�ܱ������м���a mol��I2(g)��b mol��H2(g)��c mol��HI(g)(a��b��c������0)��������Ӧ��ƽ��ʱ��HI�����������Ϊ0.60����a��b��c�Ĺ�ϵΪ________(��һ����a��b��c�Ĵ���ʽ��ʾ)��
(4)����ʱ��0.01 mol��HI��������ˮ���100 ml��Һ�������Һ����ˮ��������������ӵ����ʵ���Ũ��Ϊ________mol��L��1���������¶ȸ���Һ��pHֵ��________(������С��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009��������һ�и����꼶�ڶ����¿�����ѧ�Ծ� ���ͣ�022
��1 mol��I2(g)��2 mol��H2(g)����ij2 L�ܱ������У���һ���¶��·�����Ӧ��I2(g)��H2(g)![]() 2HI(g)����H��0�����ﵽƽ�⣮HI�����������(HI)��ʱ��仯��ͼ����(��)��ʾ��
2HI(g)����H��0�����ﵽƽ�⣮HI�����������(HI)��ʱ��仯��ͼ����(��)��ʾ��
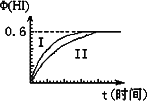
(1)��ƽ��ʱ��I2(g)�����ʵ���Ũ��Ϊ________mol��L��1��
(2)���ı䷴Ӧ��������ij��������(HI)�ı仯������(��)��ʾ���������������________(�����������������)��
�ٺ��������£������¶�
�ں��������£������¶�
�ۺ��������£���С��Ӧ�������
�ܺ��������£�����Ӧ�������
�ݺ��¡����������£������ʵ�����
(3)�������¶Ȳ��䣬����һ��ͬ��2 L�ܱ������м���a mol��I2(g)��b mol��H2(g)��c mol��HI(g)(a��b��c������0)��������Ӧ��ƽ��ʱ��HI�����������Ϊ0.60����a��b��c�Ĺ�ϵΪ________(��һ����a��b��c�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com