
ŅŌĮņĢśæó(Ö÷ŅŖ³É·ÖĪŖFeS2)ĪŖŌĮĻÖʱøĀČ»ÆĢś¾§Ģå(FeCl3·6H2O)µÄ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ
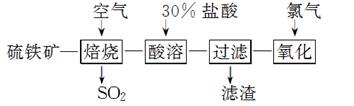
FeCl3·6H2O(¾§Ģå“ÖĘ·)
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŌŚŅ»¶ØĢõ¼žĻĀ£¬SO2×Ŗ»ÆĪŖSO3µÄ·“Ó¦ĪŖ2SO2(g)£«O2(g)??2SO3(g)£¬øĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖK£½____£»¹żĮæµÄSO2ÓėNaOHČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________________________________________________________________”£
(2)ĖįČܼ°ŗóŠų¹ż³ĢÖŠ¾łŠč±£³ÖŃĪĖį¹żĮ棬ĘäÄæµÄŹĒ________________________”¢________________________________________________________________________”£
(3)ĶØĀČĘųŃõ»ÆŹ±£¬·¢ÉśµÄÖ÷ŅŖ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ________________________________£»
øĆ¹ż³Ģ²śÉśµÄĪ²ĘųæÉÓĆ¼īČÜŅŗĪüŹÕ£¬Ī²ĘųÖŠĪŪČ¾æÕĘųµÄĘųĢåĪŖ_______________________”£
(Š“»ÆѧŹ½)”£
“š°ø””(1)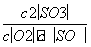 ””SO2£«NaOH===NaHSO3
””SO2£«NaOH===NaHSO3
(2)ĢįøßĢśŌŖĖŲµÄ½ž³öĀŹ””ŅÖÖĘFe3£«Ė®½ā
(3)Cl2£«2Fe2£«===2Cl££«2Fe3£« ””Cl2”¢HCl
½āĪö””(1)øł¾Ż»Æѧ·“Ó¦·½³ĢŹ½æÉŠ“³öĘ½ŗā³£Źż±ķ“ļŹ½ĪŖK£½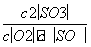 £¬¹żĮ涞Ńõ»ÆĮņÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ӧɜ³ÉŃĒĮņĖįĒāÄĘ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖSO2£«NaOH===NaHSO3”£(2)¼ÓČė¹żĮæŃĪĖįæɱ£Ö¤ĢśŌŖĖŲ½ž³öµ½ČÜŅŗÖŠ£¬ĒŅFe3£«ČŻŅ×Ė®½ā£¬¼ÓĖįæÉŅÖÖĘĘäĖ®½ā”£(3)ĶØĀČĘųµÄÄæµÄŹĒŃõ»ÆFe2£«£¬Ī²ĘųÖŠµÄÓŠŗ¦ĘųĢåŹĒŹ£ÓąµÄĀČĘųŅŌ¼°ŃĪĖį»Ó·¢³öµÄĀČ»ÆĒāĘųĢ唣
£¬¹żĮ涞Ńõ»ÆĮņÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ӧɜ³ÉŃĒĮņĖįĒāÄĘ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖSO2£«NaOH===NaHSO3”£(2)¼ÓČė¹żĮæŃĪĖįæɱ£Ö¤ĢśŌŖĖŲ½ž³öµ½ČÜŅŗÖŠ£¬ĒŅFe3£«ČŻŅ×Ė®½ā£¬¼ÓĖįæÉŅÖÖĘĘäĖ®½ā”£(3)ĶØĀČĘųµÄÄæµÄŹĒŃõ»ÆFe2£«£¬Ī²ĘųÖŠµÄÓŠŗ¦ĘųĢåŹĒŹ£ÓąµÄĀČĘųŅŌ¼°ŃĪĖį»Ó·¢³öµÄĀČ»ÆĒāĘųĢ唣



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ¹ģµĄ±ķŹ¾Ź½ÄܱķŹ¾ŃõŌ×ÓµÄ×īµĶÄÜĮæדĢ¬µÄŹĒ(””””)
A.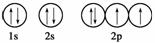
B.
C.
D.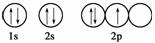
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
±µŌŚŃõĘųÖŠČ¼ÉÕŹ±µĆµ½Ņ»ÖÖ±µµÄŃõ»ÆĪļ¾§Ģ壬¾§Ģå½į¹¹ČēĶ¼ĖłŹ¾£¬ÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ(””””)
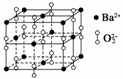
A£®øĆ¾§ĢåŹōÓŚ·Ö×Ó¾§Ģå B£®¾§ĢåµÄ»ÆѧŹ½ĪŖBa2O2

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼ĖłŹ¾ŹĒŅ»ÖÖ×ŪŗĻ“¦ĄķSO2·ĻĘųµÄ¹¤ŅÕĮ÷³Ģ£¬ČōĆæ²½¶¼ĶźČ«·“Ó¦”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
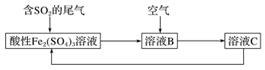
A£®ČÜŅŗBÖŠ·¢ÉśµÄ·“Ó¦ĪŖ2SO2£«O2===2SO3
B£®æÉÓĆĖįŠŌøßĆĢĖį¼ŲČÜŅŗ¼ģŃéČÜŅŗCÖŠŹĒ·ńŗ¬ÓŠFe2£«
C£®ÓÉŅŌÉĻĮ÷³ĢæÉĶĘÖŖŃõ»ÆŠŌ£ŗFe3£«>O2>SO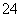
D£®“Ė¹¤ŅÕµÄÓŵćÖ®Ņ»ŹĒĪļÖŹÄÜŃ»·ĄūÓĆ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŅĶ¼±ķŹ¾Ä³¹ĢĢ¬
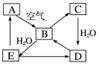
µ„ÖŹA¼°Ęä»ÆŗĻĪļÖ®¼äµÄ×Ŗ»Æ¹ŲĻµ(ijŠ©²śĪļŗĶ·“Ó¦Ģõ¼žŅŃĀŌČ„)”£»ÆŗĻĪļBŌŚ³£ĪĀ³£Ń¹ĻĀĪŖĘųĢ壬BŗĶCµÄĻą¶Ō·Ö×ÓÖŹĮæÖ®±ČĪŖ4”Ć5£¬»ÆŗĻĪļDŹĒÖŲŅŖµÄ¹¤ŅµŌĮĻ”£
(1)Š“³öAŌŚ¼ÓČČĢõ¼žĻĀÓėH2·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
________________________________________________________________________ӣ
(2)Š“³öEÓėAµÄĒā»ÆĪļ·“Ӧɜ³ÉAµÄ»Æѧ·½³ĢŹ½£ŗ
________________________________________________________________________ӣ
(3)Š“³öŅ»øöÓÉDÉś³ÉBµÄ»Æѧ·½³ĢŹ½£ŗ
________________________________________________________________________ӣ
(4)½«5 mL 0.10 mol·L£1µÄEČÜŅŗÓė10 mL 0.10 mol·L£1µÄNaOHČÜŅŗ»ģŗĻ”£
¢ŁŠ“³ö·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ_______________________________________________£»
¢Ś·“Ó¦ŗóČÜŅŗµÄpH________7(Ģī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±)£¬ĄķÓÉŹĒ________________________________________________________________________£»
¢Ū¼ÓČČ·“Ó¦ŗóµÄČÜŅŗ£¬ĘäpH________(Ģī”°Ōö“ó”±”¢”°²»±ä”±»ņ”°¼õŠ””±)£¬ĄķÓÉŹĒ________________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Įņ“śĮņĖįÄĘŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤²śĘ·”£Ä³ŠĖȤŠ”×éÄāÖʱøĮņ“śĮņĖįÄĘ¾§Ģå(Na2S2O3·5H2O)”£
¢ń.[²éŌÄ׏ĮĻ]
(1)Na2S2O3·5H2OŹĒĪŽÉ«ĶøĆ÷¾§Ģ壬Ņ×ČÜÓŚĖ®£¬ĘäĻ”ČÜŅŗÓėBaCl2ČÜŅŗ»ģŗĻĪŽ³ĮµķÉś³É”£
(2)ĻņNa2CO3ŗĶNa2S»ģŗĻČÜŅŗÖŠĶØČėSO2æÉÖʵĆNa2S2O3£¬ĖłµĆ²śĘ·³£ŗ¬ÓŠÉŁĮæNa2SO3ŗĶNa2SO4”£
(3)Na2SO3Ņ×±»Ńõ»Æ£»BaSO3ÄŃČÜÓŚĖ®£¬æÉČÜÓŚĻ”HCl”£
¢ņ.[Öʱø²śĘ·]
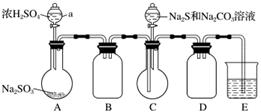
ŹµŃé×°ÖĆČēĶ¼ĖłŹ¾(Ź”ĀŌ¼Š³Ö×°ÖĆ)£ŗ
ŹµŃé²½Öč£ŗ
(1)¼ģ²é×°ÖĆĘųĆÜŠŌ£¬°“Ķ¼Ź¾¼ÓČėŹŌ¼Į”£
ŅĒĘ÷aµÄĆū³ĘŹĒ________£»EÖŠµÄŹŌ¼ĮŹĒ________(Ń”ĢīĻĀĮŠ×ÖÄø±ąŗÅ)”£
A£®Ļ”H2SO4
B£®NaOHČÜŅŗ
C£®±„ŗĶNaHSO3ČÜŅŗ
(2)ĻČĻņCÖŠÉÕĘæ¼ÓČėNa2SŗĶNa2CO3»ģŗĻČÜŅŗ£¬ŌŁĻņAÖŠÉÕĘæµĪ¼ÓÅØH2SO4”£
(3)“żNa2SŗĶNa2CO3ĶźČ«ĻūŗÄŗ󣬽įŹų·“Ó¦”£¹żĀĖCÖŠ»ģŗĻĪļ£¬ĀĖŅŗ¾__________(ĢīŠ“²Ł×÷Ćū³Ę)”¢½į¾§”¢¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ£¬µĆµ½²śĘ·”£
¢ó.[Ģ½¾æÓė·“Ė¼]
(1)ĪŖŃéÖ¤²śĘ·ÖŠŗ¬ÓŠNa2SO3ŗĶNa2SO4£¬øĆŠ”×éÉč¼ĘĮĖŅŌĻĀŹµŃé·½°ø£¬Ēė½«·½°ø²¹³äĶźÕū”£
(ĖłŠčŹŌ¼Į“ÓĻ”HNO3”¢Ļ”H2SO4”¢Ļ”HCl”¢ÕōĮóĖ®ÖŠŃ”Ōń)
Č”ŹŹĮæ²śĘ·Åä³ÉĻ”ČÜŅŗ£¬µĪ¼Ó×ćĮæBaCl2ČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬________________£¬Čō³ĮµķĪ“ĶźČ«Čܽā£¬²¢ÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢå²śÉś£¬ŌņæÉČ·¶Ø²śĘ·ÖŠŗ¬ÓŠNa2SO3ŗĶNa2SO4”£
(2)ĪŖ¼õɣװÖĆCÖŠÉś³ÉNa2SO4µÄĮ棬ŌŚ²»øıäŌӊװÖƵĻł“”ÉĻ¶ŌŹµŃé²½Öč(2)½ųŠŠĮĖøĽų£¬øĽųŗóµÄ²Ł×÷ŹĒ
________________________________________________________________________ӣ
(3)Na2S2O3·5H2OµÄČܽā¶ČĖęĪĀ¶ČÉżøßĻŌÖųŌö“ó£¬ĖłµĆ²śĘ·Ķعż________________·½·ØĢį“攣
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹¤ŅµÉĻŅŌĮņĢśæóĪŖŌĮĻÖĘĮņĖįĖł²śÉśµÄĪ²ĘųÖŠŗ¬ÓŠSO2£¬ĪŖĮĖ±ćÓŚ¼ąæŲ£¬ŹµŹ©»·¾³±£»¤£¬ĻĀĮŠŹŹŗĻ²ā¶ØĮņĖįĪ²ĘųÖŠSO2ŗ¬ĮæµÄŹŌ¼ĮŹĒ(””””)
A£®Ę·ŗģČÜŅŗ B£®µāĖ®”¢µķ·ŪČÜŅŗ
C£®°±Ė®”¢·ÓĢŖŹŌŅŗ D£®ŅŌÉĻ¶¼ÄÜ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚµŚnµē×Ó²ćÖŠ£¬µ±Ėü×÷ĪŖŌ×ÓµÄ×īĶā²ćŹ±£¬ČŻÄɵē×ÓŹż×ī¶ąÓė(n£1)²ćĻąĶ¬”£µ±Ėü×÷ĪŖŌ×ӵēĪĶā²ćŹ±£¬Ęäµē×ÓŹż±Č(n£1)²ć¶ą10øö£¬Ōņ“Ėµē×Ó²ćŹĒ(””””)
A£®K²ć B£®L²ć C£®M²ć D£®N²ć
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓŠŅ»Ļ”ĮņĖįŗĶĻ”ĻõĖįµÄ»ģŗĻĖį£¬ĘäÖŠH2SO4ŗĶHNO3µÄĪļÖŹµÄĮæÅØ¶Č·Ö±šŹĒ4 mol·L£1ŗĶ2 mol·L£1£¬Č”10 mL“Ė»ģŗĻĖį£¬ĻņĘäÖŠ¼ÓČė¹żĮæµÄĢś·Ū£¬“ż·“Ó¦½įŹųŗó£¬æɲśÉś±ź×¼×“æöĻĀµÄĘųĢåµÄĢå»żĪŖ(Éč·“Ó¦ÖŠHNO3±»»¹Ō³ÉNO)(””””)
A£®0.448 L B£®0.672 L
C£®0.896 L D£®0.224 L
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com